Figure 1.
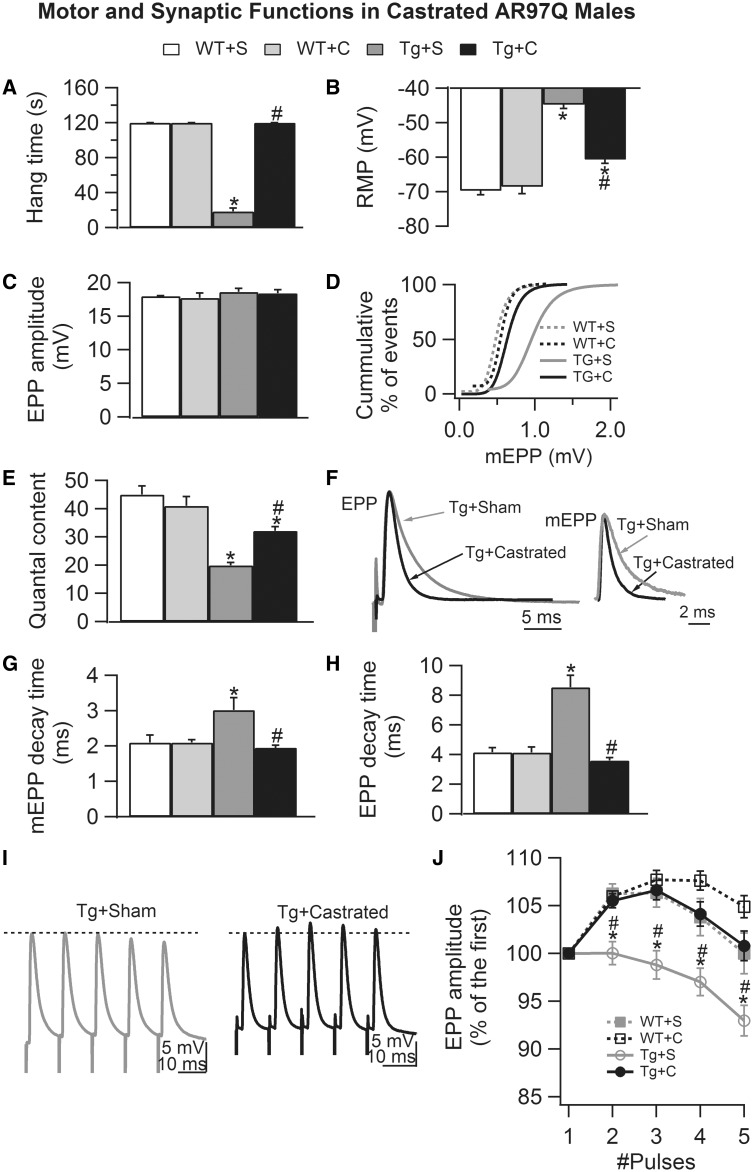
Castration of pre-symptomatic transgenic AR97Q males largely protects synaptic function. (A) Motor function based on hang times for AR97Q males was fully protected by castration pre-symptomatically, confirming previous reports. (B) The RMP of muscle fibers in symptomatic gonadally intact AR97Q males (Tg + S) is significantly depolarized compared with that of WT muscle fibers (WT + S, WT + C). Castrating pre-symptomatic Tg males largely reverses this effect of disease, maintaining membrane excitability at near normal in age-matched castrated Tg (Tg + C) males. Note however a small but significant residual effect on the RMP of AR97Q fibers, despite normal hang times of Tg + C males (A). This residual deficit may be related to the fact that fibers at the time of castration are already significantly depolarized (Fig. 3). Although neither disease or gonadal androgens affects EPP amplitude (C), the cumulative histograms of mEPP amplitude (D) show that disease shifts mEPPs toward significantly larger values (seen as a rightward shift in the curve), an effect prevented by castration (P values <0.05 on the basis of Kolmogorov–Smirnov test). (E) Likewise, disease impairs evoked transmitter release, significantly decreasing QC by more than half in gonadally intact Tg males, an effect largely but not fully prevented by castration. These data suggest that castration also rescued the size of the RRP of synaptic vesicles and/or probability of release. The residual deficit in QC in castrated Tg males may also be related to an early deficit in QC pre-symptomatically (Fig. 3). (F) Representative EPP (average of 10–20 responses) and mEPP traces (average of 2 min recording), normalized and aligned by their peak, from castrated (black) and sham (gray)-operated Tgs reveal visibly prolonged decay times for junctions from diseased gonadally intact Tg males compared with asymptomatic castrated Tg males. (G and H) Averaged values confirm significant increases in both EPP and mEPP decay times for gonadally intact, motor-impaired Tg males, an effect that is completely prevented by castration of pre-symptomatic Tg males. These data suggest that the pathological expression of the gamma subunit of the AChR is also reversed, as we later confirmed (Fig. 3). (I and J) Castration also improves short-term synaptic facilitation in Tg males, reversing a significant disease-related deficit. Representative traces of EPPs (average of 10–20 individual EPPs evoked by trains of five pulses at 100 Hz) in sham-control (black) and castrated (gray) Tg males show that significant decreases in synaptic facilitation emerge quickly, evident even at the second pulse in diseased Tg males (J). Although castration also seemed to enhance synaptic facilitation of WT junctions, this effect was not significant. Values plotted are group means (N for motor function and n for synaptic function) ± SEM (WT sham: n/N = 30/3; WT castrate: n/N = 30/3; Tg sham: n/N = 42/4; Tg castrate: n/N = 52/5) with n indicating the number of endplates/mice and N indicating the number of mice/experimental group. *P < 0.05 from WT sham or WT castrated EDL; #P < 0.05 from Tg sham EDL. ‘S’ denotes sham castration; ‘C’ denotes castration.