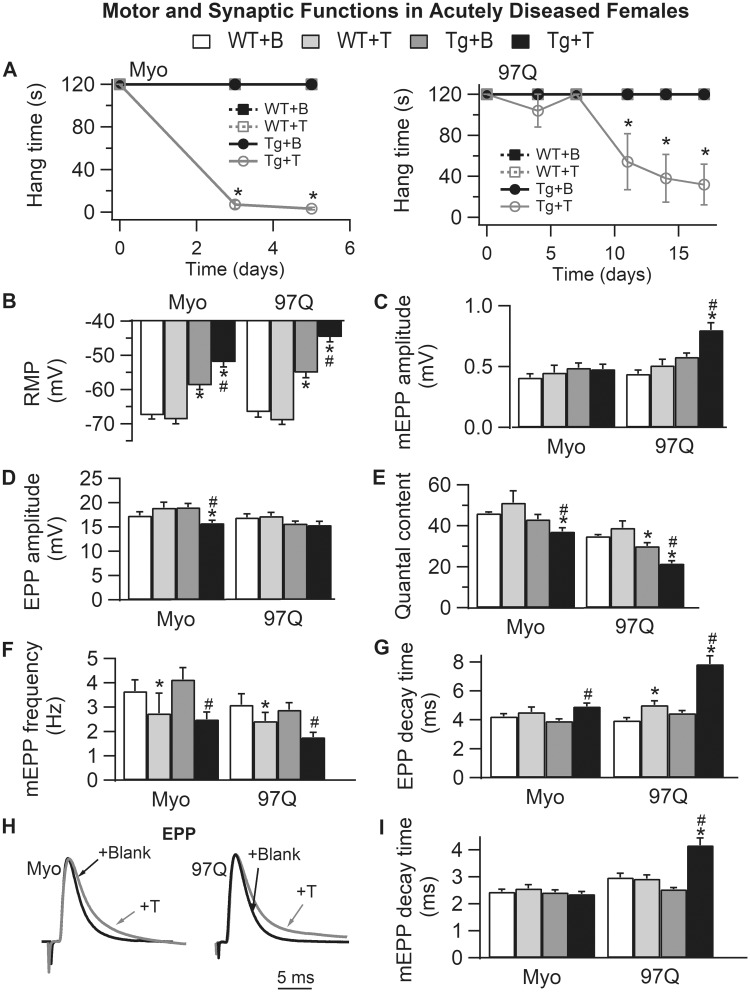
Figure 5.
Acute androgen treatment induces comparable synaptic and motor dysfunction in two female models of SBMA, the myogenic (Myo) and AR97Q models. (A) Only 5 days of testosterone treatment drives rapid loss of motor function in myogenic females (expressing WT androgen receptor only in muscle fibers), whereas androgen-dependent demise of motor function in AR97Q females takes a full 2 weeks. (B) The RMP of EDL muscle fibers is significantly depolarized in testosterone (T)-treated females of both models, comparable with that of diseased males. Note that the RMP in asymptomatic blank (B)-treated Tg females in both models is also significantly depolarized compared with WT controls, akin to that of pre-symptomatic AR97Q males (Fig. 3). These data indicate that muscle fibers in SBMA models are diseased prior to overt motor dysfunction and independent of male levels of androgens. T has no such effect on the RMP of WT fibers. (C) Only junctions in diseased AR97Q females show significant increases in mEPP amplitude triggered by T, comparable with that of symptomatic AR97Q males (Fig. 3). (D) On the other hand, EPP amplitude is significantly reduced by T treatment in myogenic females. (E) The net effect of T on the size of EPPs and mEPPs in the two models is a significant reduction in QC for symptomatic Tg females of both models. Also note that QC is significantly reduced in asymptomatic AR97Q females, again mirroring defects observed in pre-symptomatic AR97Q males. These data also underscore the possibility that some effects of mutant AR may be independent of androgens. Moreover, synaptic weakening is likely a core trait of SBMA that begins early and precedes overt motor dysfunction. (F) Androgen treatment also reduced mEPP frequency, although this effect does not depend on the disease-causing transgene, because T also has comparable effects in WTs, suggesting that this effect is not a product of disease. (G–I) T prolongs decay time of EPPs (G and H) in both Tg models, correlating with motor dysfunction, and comparable with chronically diseased males in both models. This defect may mark the progression of disease from asymptomatic to symptomatic, because it is not evident in asymptomatic Tg females nor in pre-symptomatic AR97Q males (Fig. 3). Representative average EPP traces from both T-treated (gray) and B-treated (black) Tg females which have been normalized and aligned by their peak to compare decay rates (H). Note that although mEPP decay time (I) is prolonged only in T-treated AR97Q females, T prolongs EPP decay time (G) for diseased junctions of both myogenic and AR97Q females. We also find that T treatment induced a slight, but significant prolongation of EPP decay in WT control females after two weeks of treatment but not after five days (WTs for AR97Q model versus WTs for myogenics), indicating that T can also affect EPP kinetics in WT muscle. These data show that although considerable pathology in synaptic and muscle functions is driven by the combination of androgens and a disease allele, some aspects of SBMA appear to be driven by a toxic AR independent of androgens whereas others by native androgen-dependent AR function. Values plotted are group means (N for motor function and n = synaptic function) ± SEM. Myogenic group: WT + B: n/N = 41/4 and WT + T: n/N = 29/3; Tg + B: n/N = 30/3 and Tg + T: n/N = 40/4. AR97Q group: WT + B: n/N = 29/3 and WT + T: n/N = 30/3; Tg + B: n/N = 41/4 and Tg + T: n/N = 40/4. n is the number of endplates per experimental group and N is the number of mice per experimental group. *P < 0.05 from WT +B or WT + T; #P < 0.05 from Tg + B. ‘B’ denotes Blank -treated; ‘T’ Testosterone-treated.