Abstract
Background
Today, no specific test for the diagnosis of multiple sclerosis (MS) is available due to the lack of characteristic symptoms at beginning. This circumstance also complicates estimation of disease progression. Recent findings provided evidence for early, non-lesional cerebellar damage in patients with (clinically definite) relapsing-remitting MS.
Objective
To investigate if microstructural cerebellar alterations can also serve as early structural biomarker for disease progression and conversion from clinically isolated syndrome (CIS) to MS.
Methods
46 patients diagnosed with CIS and 26 age-matched healthy controls were admitted to high-resolution MRI including diffusion tensor imaging (DTI) to examine atrophy and microstructural integrity of the cerebellum. Microstructural integrity of cerebellar white matter was assessed by fractional anisotropy (FA) as derived from DTI.
Results
Although all 46 patients of our CIS cohort showed no cerebellar lesions in structural MRI (T1w, T2w, FLAIR), their mean cerebellar FA was already reduced compared to healthy controls. Significant FA reduction at follow-up DTI 6 months after baseline examination was observed. In 16 patients that converted to MS, we found a correlation between initial cerebellar FA and conversion latency (R = 0.71, p < 0.002). Initial cerebellar FA under FAcrit = 0.352 predicted conversion into relapsing-remitting MS within 24 months (FAcrit: mean cerebellar FA of patients with early MS, determined in another study).
Conclusion
DTI seems to reflect early tissue injury in beginning MS, when atrophy and lesions are not yet detectable. Decreased cerebellar FA in patients with CIS might indicate an active and unstable disease stage, resulting in a shorter conversion time into MS.
Highlights
-
•
Convincing evidence for hidden active disease processes in patients with CIS
-
•
Widespread alterations of cerebellar white matter FA in NAWM
-
•
Decreased cerebellar FA indicated shorter conversion time into MS
-
•
New spotlight to the time point of diagnosis of multiple sclerosis
1. Introduction
Relapsing-remitting multiple sclerosis (MS) occurs with a variety of different symptoms such as vision problems, changes in sensation, and muscle weakness (Miller et al., 2005b; Sospedra and Martin, 2005). These unspecific symptoms alone do not permit the diagnosis of MS. For diagnosis of MS according to the current McDonald criteria (Polman et al., 2011) magnetic resonance imaging (MRI) detectable lesions in the central nervous system are mandatory. Patients with clinical signs alone without dissemination of lesions in space and time are classified as clinically isolated syndrome (CIS) - a potential subacute phase of MS onset (Miller et al., 2005b).
The conceptual delimitation between CIS and MS is however problematic because patients with CIS frequently already demonstrate abnormalities in normal appearing white and grey matter indicating an advanced pathological state (Miller et al., 2005a). A relevant number of patients with CIS (30–70%) develop MS, where silent MRI lesions increase the likelihood (Miller et al., 2005b). Current McDonald criteria (Polman et al., 2011) and MAGNIMS revisions (Filippi et al., 2016; Montalban et al., 2010; Rovira et al., 2009; Swanton et al., 2006) focus on the relevance of MRI criteria, such as dissemination in space and time. Especially in context with the term dissemination in time, it seems interesting to consider lesion-preceding processes. Recent studies provided evidence that microstructural tissue alterations in normal appearing white matter (NAWM), in the following briefly microstructural tissue alterations, can precede macroscopic lesions and white matter (WM) tissue atrophy in patients with MS (Deppe et al., 2014; Deppe et al., 2016a). The major problem with tissue alterations on a micron level is that conventional MRI can fail to detect them (Deppe et al., 2013). Instead, microstructure-sensitive MRI protocols like diffusion tensor imaging (DTI) are necessary. The most prominent DTI measure for quantitative assessment of microstructural alterations of NAWM based on normal values is the fractional anisotropy (FA) (Deppe et al., 2007).
The majority of recent studies on patients with MS investigated cerebral white matter, although infratentorial white matter is also affected. In the present study, we exclusively focused on cerebellar changes because infratentorial tissue alterations are of special interest due to their higher prevalence in MS compared to other inflammatory diseases (Tobyne et al., 2017) and their prognostic relevance for disability progression (Minneboo et al., 2004; Rovira et al., 2009; Wattjes et al., 2007). MS differential diagnoses like neuromyelitis optica (NMO)(Fan et al., 2017), arterial hypertension, cerebral autosomal dominant arteriopathy and Susac syndrome (Kleffner et al., 2008; Kleffner et al., 2010; Kleffner et al., 2012) show MS-like symptoms, but less cerebellar involvement.
Recently it was demonstrated by means of DTI that “normal appearing” cerebellar white matter could be damaged on a microscopic level in a very early stage of MS without presenting macroscopic lesions in T1w or T2w MRI (Deppe et al., 2016a; Deppe et al., 2016b). Interestingly, this non-lesional microstructural damage preceded cerebellar atrophy (Deppe et al., 2016b). Against this background, we hypothesized that (1) patients with CIS have already decreased cerebellar WM FA (used interchangeably: cerebellar FA). (2) The amount of decreased initial cerebellar FA has a predictive value for disease progression, namely the time to convert into (clinically definitive) MS. We further speculated that microstructural disease activity as assessed by further FA reduction at a follow-up MRI might be associated with (3) cerebellar atrophy and (4) the patients' expanded disability status scale (EDSS) – indicating an advanced disease stage and ongoing disease activity.
2. Methods
2.1. Subjects
Forty-six consecutive patients (31 f, 15 m) with a CIS and without evidence for cerebellar lesions were included in our study for high-resolution structural and diffusion weighted MRI at 3T (EDSS: range 0 to 3.5, median 1.0, lower quartile 1.0, upper quartile 2.0; disease duration: range 0.97 to 90.2 months, median 9.6 months, lower quartile 2.6 months, upper quartile 36.9, age: mean 39.3 y, range 23 y–56 y, SD 9.9 y). All 46 patients were diagnosed with CIS at their baseline MRI, 34 of them got disease-modifying therapy (Betainterferons N = 17, Glatiramer acetate N = 12, Teriflunomide N = 3, Fingolimod N = 2), the other 12 patients were treatment naive.
A subgroup of 24 patients with CIS (17 f, 7 m, EDSS: range 0 to 3.5, median 1.0, lower quartile 1.0, upper quartile 2.0; disease duration: range 1.3 to 71.8 months, median 9.6 months, lower quartile 3.8 months, upper quartile 19.8, age: mean 43.0 y, range 24 y–56 y, SD 8.7 y) was admitted to a “six months” follow-up MRI (examination interval median 181 days, lower quartile 169 days, upper quartile 206). The patients of this subgroup were also diagnosed with CIS at follow-up.
During our study (duration 4.5 y) 16 of the 46 patients converted to (clinically definite) MS (age: mean 38.5 y, range 24 y–56 y, SD 11.2 y). The conversion latency (time between first diagnosis of CIS and diagnosis of clinically definite relapsing-remitting MS) ranged from 47 days to 1458 days (median 357 days, lower quartile 227 days upper quartile 654 days). The overlap between both groups consisted of seven patients (conversion latency from CIS to MS between 191 days and 1295 days). Thirteen patients did not get a 6 months follow-up MRI and did not convert during the study. Thus, the data of these patients was only used for testing hypothesis 1. Twenty- six age-matched neurologically and psychiatrically healthy volunteers (19 f, 7 m) were included into the study as healthy control (HC) group (age: mean 37.0 y, range 23–69 years, SD 13.8 years), for details see (Deppe et al., 2016b).
Written informed consent was obtained from all study participants. The participants were also informed that the examination could reveal potentially medically significant findings and given the option to request notification in the event of such findings. The interdisciplinary committee of the University of Münster and the Westphalia-Lippe Chamber of Physicians (Ärztekammer Westfalen-Lippe) approved all examinations (ref. 2010-378-b-S).
2.2. MRI
All participants were scanned using a 3T Siemens MRI scanner and a 64-channel head/neck coil (Siemens AG, Erlangen, Germany) using the same MRI parameters and protocols: 3D T1w MP-RAGE (192 sagittal slices, FOV 256 mm, 1 mm isotropic resolution, GRAPPA 2) for cerebellum reconstruction and a diffusion weighted EPI protocol (36 axial slices, FOV 230 mm, slice thickness 3.6 mm, resolution 1.8 mm × 1.8 mm, 20 gradient directions with b = 1000 s/mm2, 5 × b = 0 s/mm2, GRAPPA 2) for DTI. Scan time for the DTI protocol was 3.5 min. In addition, a FLAIR protocol for lesion assessment was applied (192 sagittal slices, FOV 256 mm, 1 mm isotropic resolution). A 3D MP-RAGE T1-weighted after intravenous gadolinium-DTPA (diethylene triamine penta-acetic acid) 0.1 mmol/kg injection was applied only for the patients with CIS. For further details see (Deppe et al., 2016b). An experienced radiologist inspected all FLAIR images for potential macroscopic lesions. Only patients without any structural abnormalities were included in our study. In the subgroup of the 16 converted patients the conventional MRI protocol (T1w, T1w + Gd-DTPA, FLAIR) built the basis for the diagnosis of (clinically definite) MS.
2.3. DTI data post-processing
DTI data preprocessing including eddy current and movement correction, rigorous spatial registration, and smoothing has been described elsewhere (Deppe et al., 2016a; Mohammadi et al., 2010). The registration algorithm provided rigorous iterative multicontrast registration steps based on FA contrasts and b0 contrasts (b = 0 s/mm2), so that volumetric effects in the resulting normalized FA maps were negligible (Mohammadi et al., 2012). All registered diffusivity images corresponded to the MNI (Montreal Neurologic Institute) coordinate space. Smoothing of these images was performed by employing position-orientation adaptive smoothing (POAS) (Becker et al., 2012; Becker et al., 2014) using the ACID-toolbox (http://www.diffusiontools.org,) for SPM (http://www.fil.ion.ucl.ac.uk/spm/). POAS was parameterized by the following values: k* = 12, κ0 = 0.8, λ = 10, σ = 8, Ncoils = 1. A detailed explanation is given elsewhere (Becker et al., 2012; Becker et al., 2014). The cerebral and cerebellar ROIs were generated from spatially registered and averaged FA maps. Further details about image processing are given elsewhere (Deppe et al., 2016b).
2.4. Estimation of lesion load, cerebral, cerebellar and fourth ventricle volume
Prior to the automated analysis by FreeSurfer all 3D MPRAGE images were intensity inhomogeneity corrected to reduce segmentation errors using in-house software (EVAL 3.0; https://EVAL.app). Volume of the cerebellum (CBWMV), intra-cranial volume (ICV) and white matter lesion load (WMLL) were obtained from FreeSurfer (Version 5.1; http://surfer.nmr.mgh.harvard.edu/) in the same way as described elsewhere (Deppe et al., 2016b). The amount of automatically detected hypointensities in the T1w images in units of mm3 served as a measure for WMLL. WM hyperintense lesions in T2w/FLAIR images were not considered because (i) we included only patients with no apparent T2w/FLAIR lesions and (ii) the main focus of the present study was the cerebellum. From the CBWMV (unit: mm3) and the absolute ICV (unit: mm3) we calculated the relative (percentage) cerebellar WM volume as CBWMV / ICV × 100%.
2.5. Statistical analysis
Testing our hypotheses, we employed general linear models (GLM, Statistica 10, Stat Soft. Inc., www.statsoft.com) for cerebellar FA and fourth ventricular volume against the time between first and second examination. All differences in EDSS, cerebellar FA, and cerebellar volume had been linearly adjusted to a time interval between first and second examination of six months. Conversion latency had been determined and compared to cerebellar FA as determined in the first examination. WM volumes, cerebellar FA reduction, and cerebellar atrophy of CIS patients were compared between first and second examination and between healthy controls using paired t-tests. We divided our cohort into a group with short conversion latency (<730 days = 24 months) and in a second group of patients presenting long conversion latency (730–1600 days).
3. Results
3.1. Initial cerebellar FA correlates with conversion latency to MS
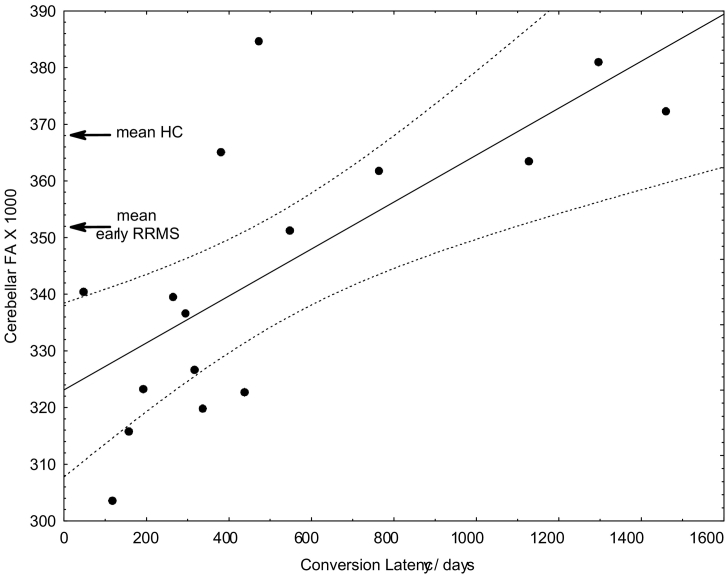
Our CIS cohort showed already at the first MRI a reduced mean cerebellar FA compared to healthy controls. The mean cerebellar FA in our present CIS collective (N = 46, mean FA = 0.336, SD = 0.024) corresponded well to the mean FA in the early MS cohort of our recent study (FA = 0.352, SD = 0.019) (Deppe et al., 2016b) and was significantly lower than in healthy controls (FA: mean: 0.368, lower quartile: 0.352, upper quartile: 0.381, SD = 0.026). In the 16 patients that converted to relapsing-remitting MS during the study, we found a significant correlation between conversion latency and cerebellar FA (linear regression analysis, depended variable: Conversion Latency, factor: Initial Cerebellar FA, regression function: Conversion Latency/days = −3722 + 12,298 × Initial Cerebellar FA, R = 0.71, p < 0.002). Interestingly, all examined patients with cerebellar FA below FAcrit = 0.352 (the mean of patients with early relapsing-remitting MS, see Deppe et al., 2016b) converted to MS within an interval of <24 months (Fig. 1). Based on this a priori critical FA value, which seemed to be representative for early relapsing-remitting MS, a corresponding contingency table revealed a sensitivity of 83% and a specificity of 100% to predict early conversion within 24 months from CIS to clinically definite MS (Table 1). Six subjects of the HC group also presented cerebellar FA below FAcrit (6 false positives), corresponding to an “over-all specificity” of 20/26 = 0.77 that a cerebellar FA below FAcrit is associated with a neurological disease.
Fig. 1.
Correlation between cerebellar white matter FA and time between first diagnosis of CIS and diagnosis of (clinically definite) MS (N = 16). The dashed curve represents the 95% confidence bands of the estimated regression function (line). For orientation, the mean cerebellar white matter FA of patients with early relapsing-remitting MS (RRMS) and the mean of healthy controls (HC) of our previous study are marked (Deppe et al., 2016b).
Table 1.
Contingency table of observed conversion latencies and cerebellar FA-based conversion predictions. The critical FA threshold of FAcrit = 0.352 is the cerebellar mean FA of patients with early RRMS as determined in our previous study (Deppe et al., 2016b). Sensitivity = TP / (TP + FN) = 10/12 = 0.83, Specificity = TN / (TN + FP) = 4/4 = 1.00, χ2 = 8.89, p = 0.0029 (TP = true positive, FN = false negative, FP = false positive, TN = true negative).
| Observation test | Early conversion (≤24 months) | Late conversion (>24 months) | |
|---|---|---|---|
| FA ≤ FAcrit (test positive) | 10 (TP) | 0 (FP) | Positive: 10 |
| FA > FAcrit (test negative) | 2 (FN) | 4 (TN) | Negative: 6 |
| 12 | 4 | 16 |
3.2. Cerebellar WM FA versus cerebral WM FA
The cerebellar FA and cerebral WM FA demonstrated a relatively strong correlation in the HC group (N = 45, RHC = 0.70, R2HC = 0.49, p < 10−6). This association between cerebral and cerebellar FA was decidedly lower (22% vs. 49% proportion of explained variance) in the patient group (N = 46, RCIS = 0.47, R2CIS = 0.22, p < 0.001).
3.3. Relative cerebellar WM volume
Consistent with our previous study (Deppe et al., 2016b), neither relative nor absolute cerebellar white matter volumes were significantly reduced in patients with CIS relative to the HC group. No significant cerebellar white or grey matter volume change within the six months interval was observed.
3.4. Cerebellar FA change between baseline and follow-up
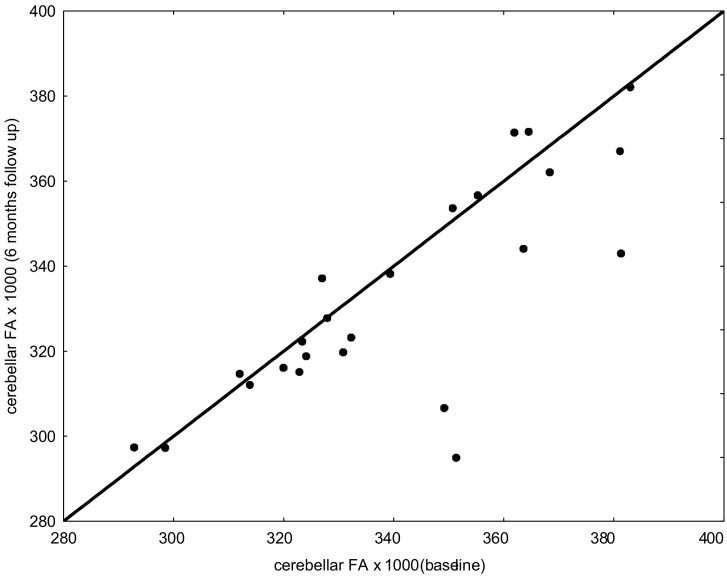
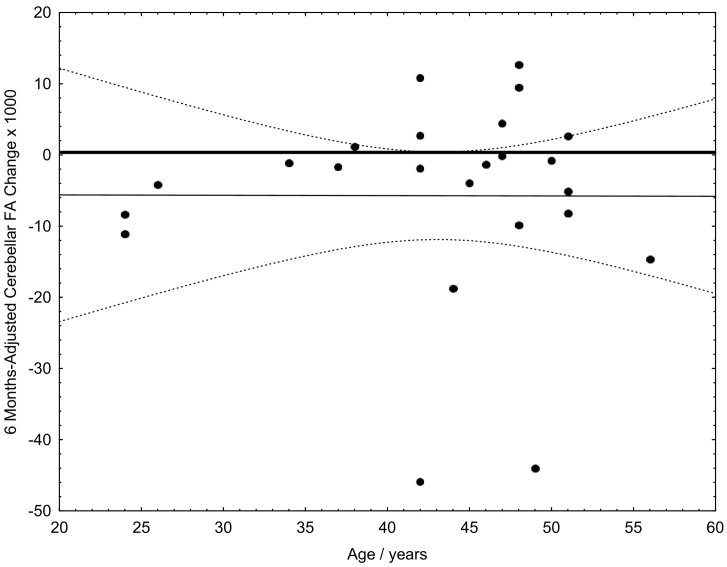
A t-test for dependent samples as well as a non-parametric Wilcoxon matched pairs test showed congruently systematic decreases of FA between baseline and follow-up MRI in the group of patients with CIS at both MRIs (N = 24, pt-test < 0.05; pWilcoxon = 0.056). The relation between first and follow-up cerebellar FA values is shown in Fig. 2. Because the interval between first and second MRI was not always exactly 6 months (see section Subjects), the FA difference had been linearly adjusted. No age or gender dependency of the 6 months FA change could be observed. For details of the individual 6 months FA differences see Fig. 3.
Fig. 2.
Cerebellar FA of first DTI (baseline) versus second DTI (six months follow-up) of the 24 patients with CIS at both examinations.
Fig. 3.
FA difference between first DTI (baseline) and follow-up. Because the interval between first and second MRI was not always exactly 6 months, the FA difference had been linearly adjusted.
3.5. Cerebellar FA reproducibility
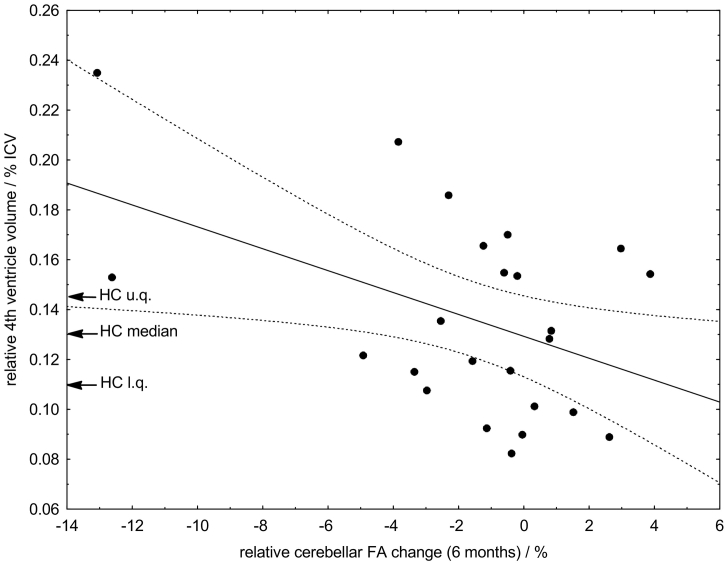
Beside the systematic FA decrease, we found a good over-all reproducibility between the baseline and six months follow-up cerebellar FA values in the patients with CIS (diagnosis of CIS also at follow up), which differed typically less than ±3% (Fig. 4). Higher FA reductions reflected relatively high volumes of the fourth ventricle in relation to the HC group. We observed a weak correlation (R = 0.46, p = 0.023) between initial fourth ventricular volume and the amount of cerebellar FA decrease between first and second examination.
Fig. 4.
Correlation between volume of the 4th ventricle and cerebellar white matter FA change during the 6 months follow-up interval.
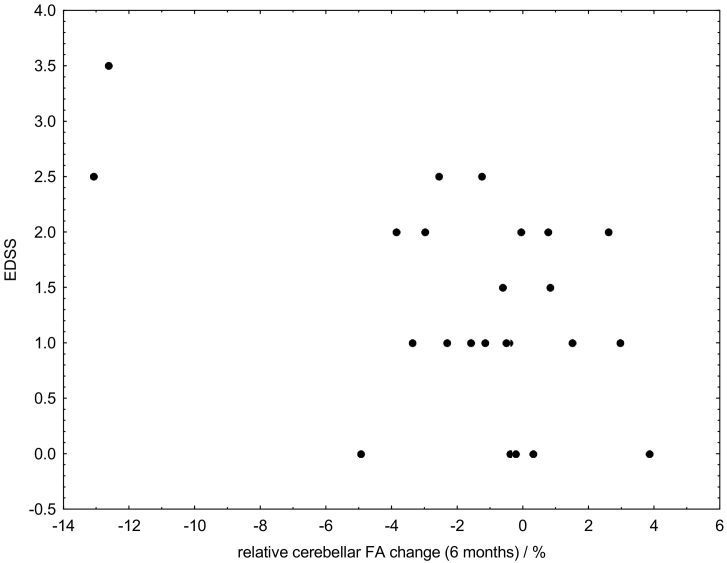
3.6. EDSS and cerebellar white matter change
A correlation between initial EDSS and cerebellar white matter change during the 6 months follow-up interval was found (N = 24, R = 0.50, p = 0.012). In none of the patients a relevant exacerbation of clinical symptoms (EDSS increase > 1 unit) was observed.
3.7. No evidence for macroscopic MRI alterations
The average volume of WM hypointensities (WMLL) did not differ between first and second examination (t = 0.89, p = 0.38) and also not between patients that converted and not converted from CIS to MS within the observation interval (t = 0.30, p = 0.77). All estimated volumes did not significantly differ from the healthy control cohort.
4. Discussion
Demyelination and neuroinflammation are of central interest in many disorders of the nervous system. However, MRI frequently fails to detect early active demyelination processes prior to lesion occurrence, as observed in a number of imaging studies involving diffusion weighted imaging (Deppe et al., 2016b). Recent studies, see introduction, underlined the importance of cerebellar changes in early MS stages to differentiate between MS and other neuroinflammatory diseases, because the latter show typically less cerebellar involvement. Here we demonstrate significantly reduced cerebellar FA in patients with an early CIS who showed no indication for cerebellar degeneration by visually inspecting conventional MRIs. In our cohort, an initial FA under FAcrit = 0.352 predicted a conversion into (clinically definite) relapsing-remitting MS within the next two years (Hypothesis 1, Fig. 1) with reasonable sensitivity and specificity (ref. Table 1). Our objective was not to derive the critical FA value (FAcrit) by the present data, but to employ an a priori threshold representative for relapsing-remitting MS. Thus, we used the mean cerebellar FA of patients with early RRMS (FARRMS = 0.352) as determined in our previous study on cerebellar FA (Table 2, Deppe et al., 2016b). All our “early converters” demonstrated an initial cerebellar FA below FAcrit and seemed to have already microstructural white matter alterations typical for early (relapsing-remitting) MS. Thus, our study sets a new spotlight to the time point of diagnosis. The group of patients with short conversion latency appeared to undergo a more active disease progression.
Some methodological issues should be addressed with respect to this study. First, we would need the verification of the observed effects in larger samples of patients with CIS. Second, methodological factors, such as scanner characteristics, head coil properties, employed DTI software, patient motion, partial volume effects, and motion artifacts caused by pulsation of the cerebrospinal fluid, might also influence the reproducibility and comparability of data. However, the present findings on the 24 patients that were examined twice demonstrate reasonable reproducibility of cerebellar FA values by the present, clinically applicable setting. In most of the patients the FA values differed less than ±3% between first and second DTI (ref. Fig. 2, Fig. 4). All higher FA discrepancies reflected always FA reductions that in turn were mainly associated with other clinical signs (increased EDSS) or short conversion times.
Why did we restrict our presented results mainly to the cerebellum and did not discuss the cerebellar alterations in context of cerebral white matter FA? According to the considerations given in the introduction, the first reason was that this study was intended as direct follow-up and validation of our previous findings on patients with early MS, now with the focus on patients with even earlier (subclinical) MS. The second reason was, that in a tentative analysis of cerebral FA, it turned out that different WM regions (e.g. corpus callosum, juxtacortical WM) have different predictive power, while the average over-all cerebral WM FA was less sensitive than the cerebellar FA. Thus, a systematic study on the effective predictive power of early cerebral WM FA reductions should not be restricted to the average cerebral WM but must account for the heterogeneous nature and sensitivity/specificity of cerebral WM. Such a thoroughgoing investigation with many regions of interest would need a much larger cohort of patients with CIS to account for the higher number of observables. The third reason was that we think that in MS infratentorial WM should be considered with special focus per se, because the integrity seemed to be much more decoupled between cerebral and cerebellar WM in patients with CIS (MS) compared to HC (compare section Cerebellar WM FA versus Cerebral WM FA: R2HC = 0.49, R2CIS = 0.22).
While the FA had been established as a robust microstructure sensitive MRI marker in a vast amount of recent DTI studies, other diffusion parameters might be also interesting for future research. Translational research investigating axial diffusivity (AD) and radial diffusivity (RD) in combination with histological findings are from a theoretical point of view more predestinated to distinguish different underlying disease mechanisms and tissue alterations. In this context, it should be pointed out that currently all diffusion parameters are not specific for MS or other inflammatory diseases. They generally only reflect changes in water mobility that might be caused by (pathological) tissue changes caused by demyelination or neurodegeneration on the micron scale. Thus, changes or abnormalities in diffusion parameters, especially FA reductions, should always be interpreted in context with clinical signs and a tentative diagnosis. In other words, if the cerebellar FA of a (healthy) volunteer or patient with CIS is below FAcrit, it does not automatically mean that this subject will convert into MS in the next future (compare 77% “over-all specificity” without neurological findings). All results shown in Fig. 1 and Table 1 refer exclusively on patients with an actually beginning MS that was only transiently diagnosed as CIS at the time of DTI.
Our study design and our results did also not permit to predict a “stable CIS course”, i.e. no conversion into MS over a (life-) long period, because we compared only patients (N = 16) that actually converted into MS during our study according to their individual conversion latencies. To target the question about predicting stable CIS courses, further research would be necessary that employs DTI for long-term observations to compare actual (long-term/lifelong) “non-converters” with “converters”.
Given that the pathological processes exacerbate by time, we linearly adjusted the temporal FA change between MRI check-ups to a 6 months interval in order to account for different observation intervals. We further could exclude any confounding effects by age on the 6-months FA change (Fig. 3). It has previously been difficult to validate directly the connection between FA, microstructural alterations, and exacerbation of clinical symptoms due to a lack of alternative (in vivo) gold standard methods providing similar data in humans, as well as the relative paucity of animal imaging data. In addition, it should be noted that neither our study nor any others of which we are aware have produced insights into how inflammation related biochemical processes, demyelination, and MRI measures actually interrelate. However, the found 6 months FA changes might most likely reflect functionally relevant alterations of the nervous system, because we found a relation between patients' EDSS and 6 months cerebellar FA reduction (Fig. 5).
Fig. 5.
Relation between EDSS and relative cerebellar white matter change during the 6 months follow-up interval.
The importance of fourth ventricular enlargement as one of the earliest MRI predictors for MS disease progression has been observed in a number of imaging studies (Lukas et al., 2010; Schneider et al., 2017). The abnormalities of fourth ventricular volume exclusively appeared in patients with MS prior to symptom occurrence and lesion visibility in T2 MRI (Lepore et al., 2013) compared to healthy controls (Schneider et al., 2017). These findings inspired our third hypothesis that disease activity as assessed by FA change might correlate with cerebellar atrophy. This hypothesis was supported by a correlation between cerebellar FA and fourth ventricular volume (Fig. 4). A higher initial volume compared to healthy controls as sign of early cerebellar atrophy corresponded to a higher rate of cerebellar FA change and shorter conversion time into (clinically definite) MS. Interestingly, cerebellar FA changed throughout the 6 months period as sign of disease progression, whereas fourth ventricular volume remained unchanged. With regard to our findings, it seems to be important to emphasize that both, cerebellar FA and fourth ventricular volume, appeared to be suitable early markers for MS. Note, however, that only cerebellar FA seemed to be sensitive enough to reflect dynamic changes within a 6 months interval and thus might indicate disease progression. It is very likely that this progression might cause the symptoms in the near future required for the diagnosis of relapsing-remitting MS.
Our findings might be relevant for future research regarding the importance of expanding diagnostic criteria towards inclusion of microstructural alterations when macroscopic alterations are not yet detectable. DTI seems to be a valid and reliable tool to detect changes on a microscopic scale that are not always detectable by conventional MRI techniques. To further investigate these findings, patients with initial symptoms alone should be admitted to DTI when receiving their first MRI. The initial microstructural status can help to classify the underlying disease mechanism and therefor accelerate the start of adequate medical treatment.
5. Conclusion
Patients with CIS who exhibit no abnormality using conventional MRI show evidence of widespread alterations of cerebellar white matter FA, suggestive of a loss of local nerve fiber integrity. The ability to detect and visualize these microstructural changes could have significant implications for the study of patients in early MS stages and for investigation of disorders associated with water diffusion abnormalities. Therefore, patients with decreased initial cerebellar FA and increased fourth ventricle volume might be expected to show greater disease activity. The present results raise but do not answer a more general question concerning the relevance of particular imaging markers for the diagnosis of MS. Further research is required to determine the etiology of microstructural alterations to permit an earlier date of diagnosis and an advanced start of medical treatment. Finally, this study provides convincing evidence for the hypothesis of hidden active disease processes in patients with CIS by a mismatch between macroscopic white matter lesions and microstructural alterations.
Conflict of interest
The authors report no potential conflicts of interest, including financial interests, activities, relationships, and affiliations.
Acknowledgement
Michael Deppe, PhD, had full access to all data in the study and takes responsibility for the data integrity and the accuracy of the data analysis. The authors thank Julia Krämer, Jan-Gerd Tenberge, Sven G. Meuth, Heinz Wiendl and Patrick Schiffler for their support of the study.
Footnotes
Authors and their individual contributions to the manuscript: Michael Deppe: Concept and idea of the study, development of MRI data analysis software, diffusion MRI data analysis, confirmatory statistical analysis, interpretation of the data, revising of manuscript, over-all supervision, organization, and funding of the project. Alexa Kugler: Recruitment of healthy control subjects, data integration, structural MRI data analysis, confirmatory statistical analysis, interpretation of the data, drafting of manuscript.
References
- Becker S.M., Tabelow K., Mohammadi S., Weiskopf N., Polzehl J. Adaptive smoothing of multi-shell diffusion weighted magnetic resonance data by msPOAS. NeuroImage. 2014;95:90–105. doi: 10.1016/j.neuroimage.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.M., Tabelow K., Voss H.U., Anwander A., Heidemann R.M., Polzehl J. Position-orientation adaptive smoothing of diffusion weighted magnetic resonance data (POAS) Med. Image Anal. 2012;16:1142–1155. doi: 10.1016/j.media.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Deppe M., Duning T., Mohammadi S., Schwindt W., Kugel H., Knecht S., Ringelstein E.B. Diffusion-tensor imaging at 3 T: detection of white matter alterations in neurological patients on the basis of normal values. Investig. Radiol. 2007;42:338–345. doi: 10.1097/01.rli.0000261935.41188.39. [DOI] [PubMed] [Google Scholar]
- Deppe M., Kramer J., Tenberge J.G., Marinell J., Schwindt W., Deppe K., Groppa S., Wiendl H., Meuth S.G. Early silent microstructural degeneration and atrophy of the thalamocortical network in multiple sclerosis. Hum. Brain Mapp. 2016;37:1866–1879. doi: 10.1002/hbm.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M., Marinell J., Kramer J., Duning T., Ruck T., Simon O.J., Zipp F., Wiendl H., Meuth S.G. Increased cortical curvature reflects white matter atrophy in individual patients with early multiple sclerosis. Neuroimage Clin. 2014;6:475–487. doi: 10.1016/j.nicl.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M., Muller D., Kugel H., Ruck T., Wiendl H., Meuth S.G. DTI detects water diffusion abnormalities in the thalamus that correlate with an extremity pain episode in a patient with multiple sclerosis. Neuroimage Clin. 2013;2:258–262. doi: 10.1016/j.nicl.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M., Tabelow K., Kramer J., Tenberge J.G., Schiffler P., Bittner S., Schwindt W., Zipp F., Wiendl H., Meuth S.G. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult. Scler. 2016;22:73–84. doi: 10.1177/1352458515579439. [DOI] [PubMed] [Google Scholar]
- Fan M., Fu Y., Su L., Shen Y., Wood K., Yang L., Liu Y., Shi F.D. Comparison of brain and spinal cord magnetic resonance imaging features in neuromyelitis optica spectrum disorders patients with or without aquaporin-4 antibody. Mult. Scler. Relat. Disord. 2017;13:58–66. doi: 10.1016/j.msard.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A., Ciccarelli O., De S.N., Evangelou N., Kappos L., Rovira A., SastreGarriga J., Tintore M., Frederiksen J.L., Gasperini C., Palace J., Reich D.S., Banwell B., Montalban X., Barkhof F. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303. doi: 10.1016/S1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffner I., Deppe M., Mohammadi S., Schiffbauer H., Stupp N., Lohmann H., Young P., Ringelstein E.B. Diffusion tensor imaging demonstrates fiber impairment in Susac syndrome. Neurology. 2008;70:1867–1869. doi: 10.1212/01.wnl.0000280580.95671.01. [DOI] [PubMed] [Google Scholar]
- Kleffner I., Deppe M., Mohammadi S., Schwindt W., Sommer J., Young P., Ringelstein E.B. Neuroimaging in Susac's syndrome: focus on DTI. J. Neurol. Sci. 2010;299:92–96. doi: 10.1016/j.jns.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Kleffner I., Duning T., Lohmann H., Deppe M., Basel T., Promesberger J., Dorr J., Schwindt W., Ringelstein E.B. A brief review of Susac syndrome. J. Neurol. Sci. 2012;322:35–40. doi: 10.1016/j.jns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Lepore S., Waiczies H., Hentschel J., Ji Y., Skodowski J., Pohlmann A., Millward J.M., Paul F., Wuerfel J., Niendorf T., Waiczies S. Enlargement of cerebral ventricles as an early indicator of encephalomyelitis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C., Minneboo A., de G. V., Moraal B., Knol D.L., Polman C.H., Barkhof F., Vrenken H. Early central atrophy rate predicts 5 year clinical outcome in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2010;81:1351–1356. doi: 10.1136/jnnp.2009.199968. [DOI] [PubMed] [Google Scholar]
- Miller D., Barkhof F., Montalban X., Thompson A., Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part 2: non-conventional MRI, recovery processes, and management. Lancet Neurol. 2005;4:341–348. doi: 10.1016/S1474-4422(05)70095-8. [DOI] [PubMed] [Google Scholar]
- Miller D., Barkhof F., Montalban X., Thompson A., Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005;4:281–288. doi: 10.1016/S1474-4422(05)70071-5. [DOI] [PubMed] [Google Scholar]
- Minneboo A., Barkhof F., Polman C.H., Uitdehaag B.M., Knol D.L., Castelijns J.A. Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch. Neurol. 2004;61:217–221. doi: 10.1001/archneur.61.2.217. [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Keller S.S., Glauche V., Kugel H., Jansen A., Hutton C., Floel A., Deppe M. The influence of spatial registration on detection of cerebral asymmetries using voxel-based statistics of fractional anisotropy images and TBSS. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S., Moller H.E., Kugel H., Muller D.K., Deppe M. Correcting eddy current and motion effects by affine whole-brain registrations: evaluation of three-dimensional distortions and comparison with slicewise correction. Magn. Reson. Med. 2010;64:1047–1056. doi: 10.1002/mrm.22501. [DOI] [PubMed] [Google Scholar]
- Montalban X., Tintore M., Swanton J., Barkhof F., Fazekas F., Filippi M., Frederiksen J., Kappos L., Palace J., Polman C., Rovaris M., De S.N., Thompson A., Yousry T., Rovira A., Miller D.H. MRI criteria for MS in patients with clinically isolated syndromes. Neurology. 2010;74:427–434. doi: 10.1212/WNL.0b013e3181cec45c. [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O'Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira A., Swanton J., Tintore M., Huerga E., Barkhof F., Filippi M., Frederiksen J.L., Langkilde A., Miszkiel K., Polman C., Rovaris M., Sastre-Garriga J., Miller D., Montalban X. A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch. Neurol. 2009;66:587–592. doi: 10.1001/archneurol.2009.49. [DOI] [PubMed] [Google Scholar]
- Schneider R., Bellenberg B., Kleiter I., Gold R., Koster O., Weiler F., Hahn H., Lukas C. Cervical cord and ventricle affection in neuromyelitis optica. Acta Neurol. Scand. 2017;135:324–331. doi: 10.1111/ane.12601. [DOI] [PubMed] [Google Scholar]
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Swanton J.K., Fernando K., Dalton C.M., Miszkiel K.A., Thompson A.J., Plant G.T., Miller D.H. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J. Neurol. Neurosurg. Psychiatry. 2006;77:830–833. doi: 10.1136/jnnp.2005.073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobyne S.M., Ochoa W.B., Bireley J.D., Smith V.M., Geurts J.J., Schmahmann J.D., Klawiter E.C. Cognitive impairment and the regional distribution of cerebellar lesions in multiple sclerosis. Mult. Scler. 2017;1352458517730132 doi: 10.1177/1352458517730132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattjes M.P., Lutterbey G.G., Gieseke J., Traber F., Klotz L., Schmidt S., Schild H.H. Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am. J. Neuroradiol. 2007;28:54–59. [PMC free article] [PubMed] [Google Scholar]