Abstract
Chronic obstructive pulmonary disease (COPD) is estimated to be the third leading cause of death by 2030. Transcription factor NF-κB may play a critical role in COPD pathogenesis. Ribosomal protein S3 (RPS3), a 40S ribosomal protein essential for executing protein translation, has recently been found to interact with the NF-κB p65 subunit and promote p65 DNA-binding activity. We sought to study whether RPS3 gene silencing could protect against cigarette-smoke (CS)-induced acute lung injury in a mouse model. Effects of an intratracheal RPS3 siRNA in CS-induced lung injury were determined by measuring bronchoalveolar lavage (BAL) fluid cell counts, levels of inflammatory and oxidative damage markers, and NF-κB translocation. Lung RPS3 level was found to be upregulated for the first time with CS exposure, and RPS3 siRNA blocked CS-induced neutrophil counts in BAL fluid. RPS3 siRNA suppressed CS-induced lung inflammatory mediator and oxidative damage marker levels, as well as nuclear p65 accumulation and transcriptional activation. RPS3 siRNA was able to disrupt CS extract (CSE)-induced NF-κB activation in an NF-κB reporter gene assay. We report for the first time that RPS3 gene silencing ameliorated CS-induced acute lung injury, probably via interruption of the NF-κB activity, postulating that RPS3 is a novel therapeutic target for COPD.
Keywords: ribosomal protein S3, chronic obstructive pulmonary disease, NF-KB, siRNA, cigarette smoke
Introduction
Chronic obstructive pulmonary disease (COPD) is an airway inflammatory disease characterized by progressive airflow limitation, increased macrophage and neutrophil infiltration, and hypersecretion of inflammatory mediators, as well as oxidant and antioxidant dysregulation.1, 2 It is estimated that more than 200 million people are suffering from COPD globally, and COPD accounts for about 3.4% of the total health care cost in the European Union and $50 billion in the United States.3 We have very few treatment options for COPD, and we mainly rely on corticosteroids and phosphodiesterase-4 (PDE4) inhibitor as anti-inflammatory agents, both of which come with dose-limiting adverse effects.4 There is an urgent need to discover novel therapies for COPD.
Nuclear factor κB (NF-κB), a master switch for pro-inflammatory gene transcription, may play a vital role in the pathogenesis of COPD. Elevated NF-κB activities have been observed in bronchial biopsies and sputum macrophages from COPD patients.5, 6 Cigarette smoke (CS) is a rich source of oxidants and free radicals and the predominant risk factor for COPD, causing oxidative damage, inflammatory cell infiltration, and imbalance of oxidants and antioxidants in the lungs.7, 8 Nuclear translocation of NF-κB p65 subunit and NF-κB transcriptional activities were upregulated in human lung epithelial cells exposed to cigarette smoke extract (CSE).9, 10 In CS-induced experimental COPD models showing airway inflammation and tissue oxidative damage, increased NF-κB nuclear accumulation in lung tissues was observed.11, 12 Cumulative evidence points to the NF-κB pathway as a target for pharmacological intervention for COPD therapy.
Ribosome is the enzyme complex that catalyzes protein synthesis, consisting of a small 40S subunit and a large 60S subunit. Ribosomal protein S3 (RPS3), a component of the small 40S ribosomal subunit, is known to be involved in the initiation of mRNA translation via its interaction with the eukaryotic initiation factors eIF-2 and eIF-3.13 Nevertheless, recent reports have unraveled an extra-ribosomal function of RPS3 as an integral part of the NF-κB complex.14, 15 RPS3 was found to bind to the p65 subunit of the NF-κB, promote the nuclear accumulation of NF-κB complex, and enhance the binding affinity of NF-κB to the κB sites of a number of target genes. In addition, we have recently reported that gene silencing of RPS3 attenuated inflammation in a house dust mite asthma mouse model.16
Small interfering RNA (siRNA), a synthetic RNA duplex consisting of usually 21-mer oligonucleotides, is designed to specifically knock down target mRNA.17 Gene silencing by siRNA is a highly target-sequence-specific process, and synthetic siRNA can be delivered via inhalation route into the airway of experimental animal models to silence the target gene of interest.18 The lung has a large absorption surface area and is lined with surfactants that can facilitate siRNA uptake into the lung cells.15 The objective of the present study was to investigate whether gene silencing of RPS3 in vivo could protect against CS-induced acute lung injury in a mouse model.
Results
In Vitro Characterization of RPS3 siRNA
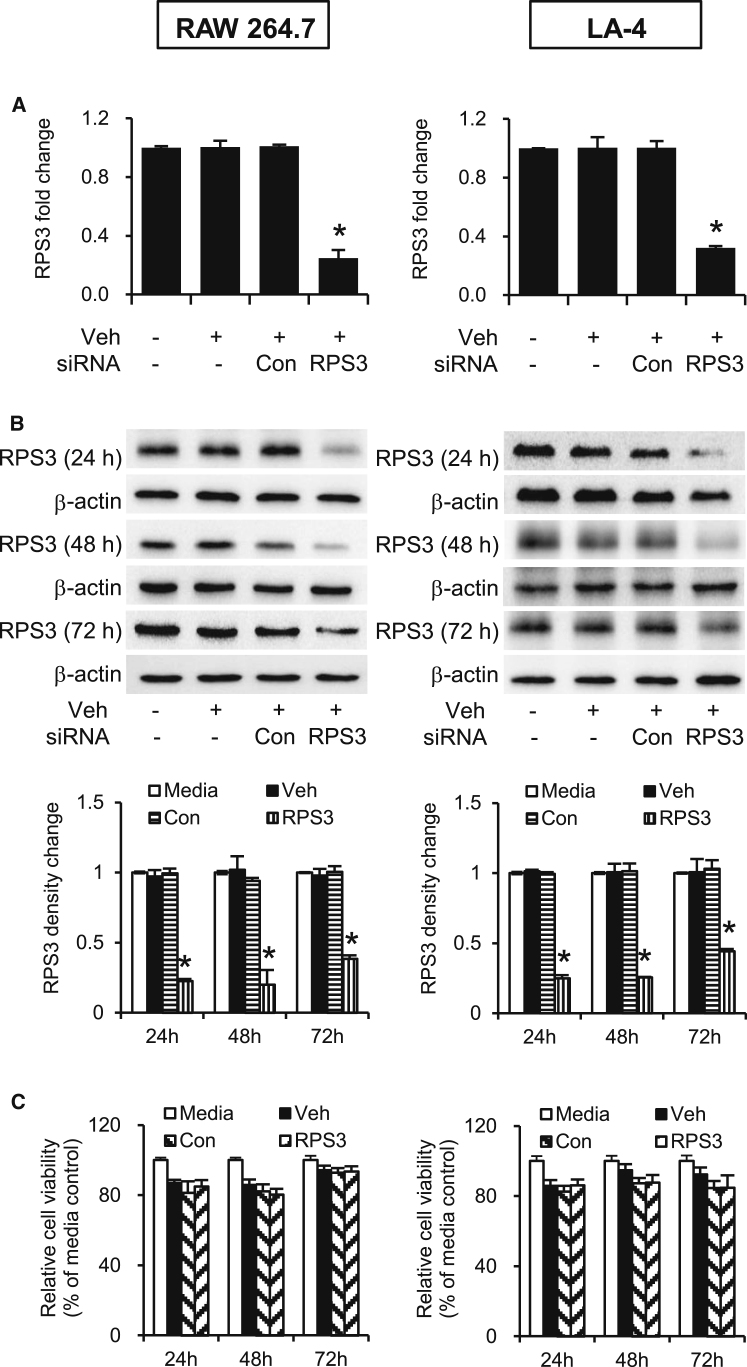
The gene silencing effects of RPS3 siRNA were confirmed in RAW 264.7 and LA-4 cells by real-time qPCR and immunoblotting. RPS3 siRNA markedly silenced RPS3 mRNA expression by about 70% in both cell lines, as compared to the control siRNA (Figure 1A). RPS3 protein expression was reduced by at least 60%–80% in both cell lines (Figure 1B). An MTS assay reveals that RPS3 siRNA had no negative impact on the proliferation of cell lines tested, as compared with control siRNA (Figure 1C).
Figure 1.
Gene Silencing Effects of RPS3 siRNA on RPS3 mRNA and Protein Levels in RAW 264.7 and LA-4 Cells
(A) Total mRNAs were extracted 24 hr after siRNA transfection (n = 3 separate experiments). (B) Total protein lysates were prepared 24, 48, and 72 hr after siRNA transfection (n = 3 separate experiments per time point). The immunoblot band intensities were analyzed using ImageJ software and normalized to endogenous control β-actin. (C) Effects of RPS3 siRNA on cell viability was detected by MTS assay (n = 6 separate experiments). Veh, Lipofectamine 2000; Con, control siRNA. Values are expressed as means of triplicate ± SEMs. *Significant difference from control siRNA, p < 0.05.
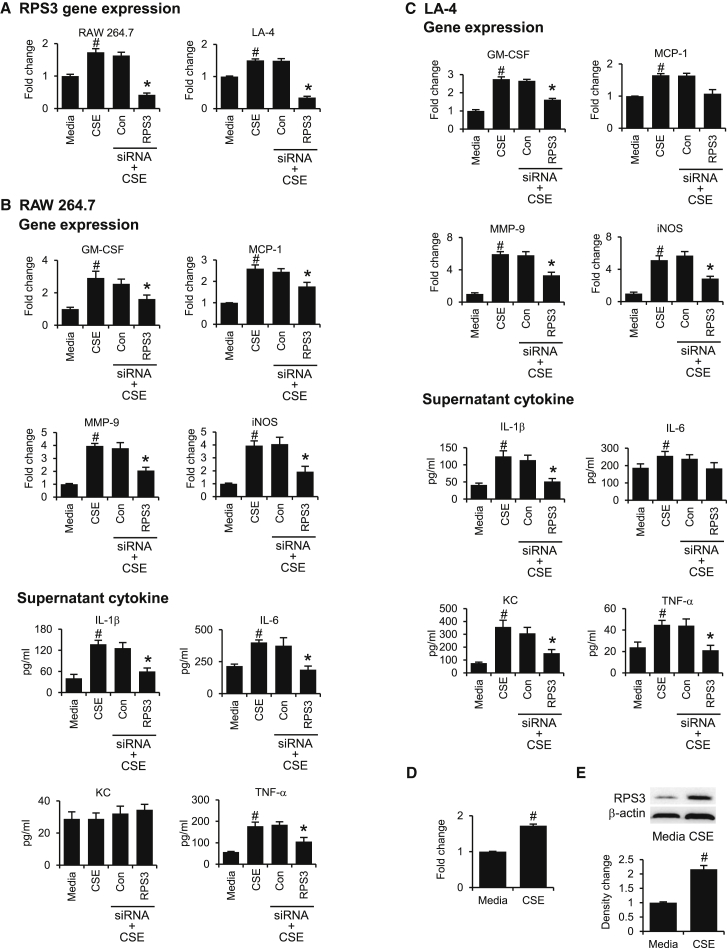
We observed an upregulation of RPS3 mRNA expression in CSE-stimulated cell lines, which can be significantly suppressed by RPS3 siRNA (Figure 2A). CSE-induced gene expression of GM-CSF (granulocyte-macrophage colony-stimulating factor), MMP-9 (matrix metallopeptidase 9), and iNOS (inducible nitric oxide synthase) in RAW 264.7 cells (Figure 2B) and LA-4 cells (Figure 2C) was significantly abated by RPS3 gene silencing. MCP-1 was increased in both cell lines upon CSE exposure and was only significantly decreased by RPS3 siRNA in RAW 264.7 cells. In addition, RPS3 siRNA markedly suppressed CSE-induced increases in supernatant levels of cytokines including interleukin (IL)-1β and tumor necrosis factor alpha (TNF-α) in both cell lines, IL-6 in RAW 264.7 cells, and keratinocyte chemokine (KC) in LA-4 cells (Figures 2B and 2C). Notably, KC was not increased by CSE in RAW 264.7 cells. IL-6 was not significantly reduced by RPS3 siRNA in LA-4 cells.
Figure 2.
Gene Silencing Effects of RPS3 siRNA on CSE-Induced RPS3 and Inflammatory Biomarker Expressions In Vitro
(A) CSE significantly increased RPS3 mRNA expression in RAW 264.7 and LA-4 cell lines, and the RPS3 mRNA levels were significantly downregulated by RSP3 siRNA (n = 6 separate experiments). Gene silencing by RPS3 siRNA significantly decreased CSE-induced gene expression of inflammatory markers in RAW 264.7 cells (B) and LA-4 cells (C). In addition, RPS3 siRNA significantly suppressed CSE-induced increases in supernatant levels of cytokines in RAW 264.7 cells (B) and LA-4 cells (C) (n = 6 separate experiments). CSE upregulated RPS3 mRNA level (D) and RPS3 protein level (E) in BEAS-2B cells (n = 6 separate experiments). Values are shown as means of duplicate ± SEMs of 6 separate experiments. *Significant difference from control siRNA (Con), p < 0.05; #significant difference from control media group, p < 0.05.
To ensure that CSE-induced upregulation of RPS3 expression in mouse cell lines is similarly observable in human airway cells, we are able to demonstrate that RPS3 mRNA (Figure 2D) and RPS3 protein (Figure 2E) were upregulated in CSE-stimulated BEAS-2B human bronchial epithelial cells, indicating a potential relevance of RPS3 in human COPD.
In Vivo Characterization of RPS3 siRNA
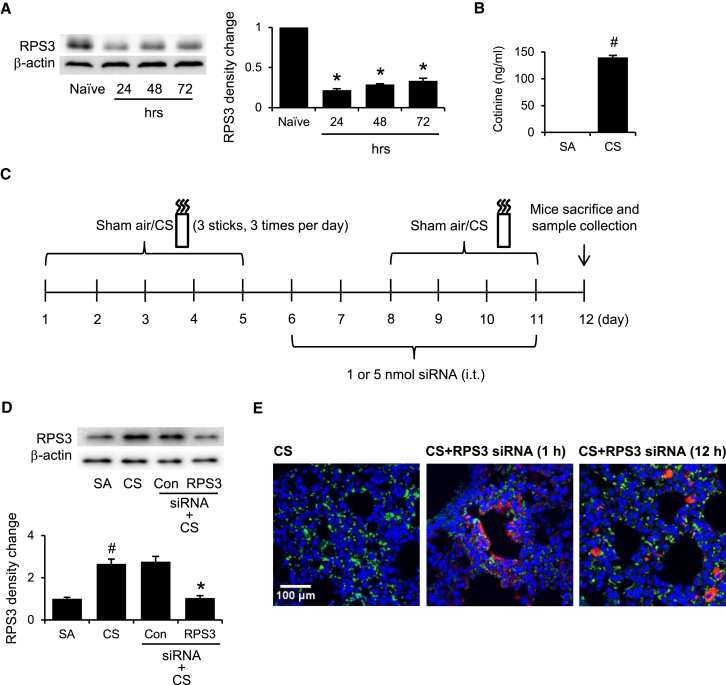
After 3 consecutive daily intratracheal administrations of 5 nmol RPS3 siRNA to naive mice, lung RPS3 protein levels were knocked down for up to 72 hr (Figure 3A). In mice exposed to CS, plasma cotinine levels were sharply elevated, as compared with those of the sham-air control group (Figure 3B). A 2-week CS-induced acute lung injury mouse model was established according to our previous report.16 Based on our preliminary study, we gave six consecutive daily intratracheal doses of RPS3 siRNA to CS-exposed mice to silence the RPS3 gene in the lungs (Figure 3C). We observed for the first time that CS exposure significantly raised the lung RPS3 protein level, and RPS3 siRNA significantly reduced the CS-induced RPS3 protein level to the basal level (Figure 3D).
Figure 3.
Regulation of RPS3 Levels by CS and Characterization of RPS3 siRNA In Vivo
(A) Representative immunoblots showing the duration of gene silencing effects of RPS3 siRNA (5 nmol) in mouse lungs for 24, 48, and 72 hr after intratracheal administration (n = 3 mice per group). Naive mice are mice without CS exposure. (B) Comparison of serum cotinine levels between sham-air-exposed (SA) and CS-exposed (CS) mice (n = 3 mice per group). (C) A 2-week CS-induced acute lung injury mouse model based on our previous report.41 Mice were given six consecutive daily intratracheal doses of RPS3 siRNA to silence the RPS3 gene in the lungs. (D) Gene silencing effects of RPS3 siRNA (5 nmol) in CS-exposed mouse lungs (n = 6 mice per group). Control siRNA (Con) refers to 5 nmol non-targeting siRNA. (E) Distribution of Cy5-labeled RPS3 siRNA (5 nmol, red) in CS-exposed mouse lungs 1 hr and 12 hr after intratracheal siRNA administration (n = 3 mice per group). Lung sections were prepared and probed with Alexa Fluor 488-conjugated anti-CD68 antibody and DAPI. Values are shown as means of triplicate ± SEMs. *Significant difference from control siRNA, p < 0.05; #significant difference from the SA group, p < 0.05.
In an attempt to visualize the distribution of intratracheally administrated siRNA in the lungs, mice were exposed to CS and then administered with Cy5-labeled RPS3 siRNA. At 1 hr and 12 hr after siRNA instillation, lung sections were probed with Alexa Fluor 488-labeled anti-CD68 antibody to detect macrophages. Red-labeled siRNA was detected on the airway epithelial layer in a band-like deposition pattern 1 hr after administration. Green-labeled macrophages were found clustered around the deposited siRNA. Twelve hours later, the red dots of siRNA were noticeable beneath the airway basement membrane and co-localized with submucosal macrophages, indicating that siRNA diffused through the epithelial barrier and endocytosed by macrophages (Figure 3E).
RPS3 Gene Silencing Attenuates CS-Induced Airway Inflammation
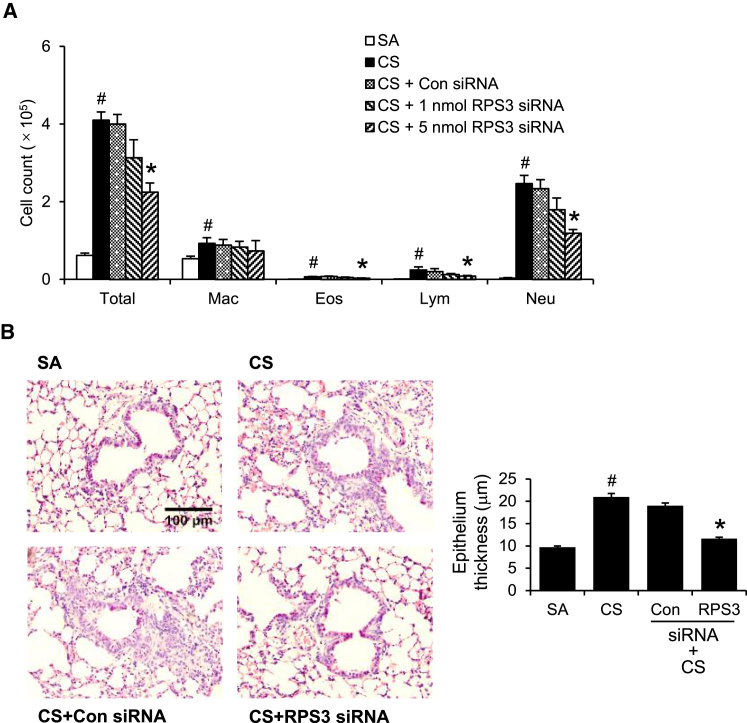
CS exposure significantly increased total cell, macrophage, neutrophil, eosinophil, and lymphocyte counts in bronchoalveolar lavage (BAL) fluid, as compared with the sham-air control (Figure 4A). Intratracheal RPS3 siRNA at 5 nmol significantly reduced total cell, neutrophil, lymphocyte, and eosinophil counts in BAL fluid, as compared with control siRNA (5 nmol) (Figure 4A). Noticeably, macrophage counts were not reduced by RPS3 siRNA. Histological analyses revealed significant inflammatory cell infiltration and epithelium thickening in CS-challenged mouse lungs. Epithelium thickness was quantified with a total of 4 additional mice per treatment group and 10 images per mouse. RPS3 gene silencing significantly reduced epithelium thickness compared with control siRNA (Figure 4B). Six doses of RPS3 siRNA treatment did not alter peripheral blood counts or kidney and liver enzyme levels (Table S1).
Figure 4.
Anti-inflammatory Effects of RPS3 siRNA in CS-Induced Acute Lung Injury In Vivo
(A) BAL fluid total and differential cell counts from CS-exposed (CS) mice with RPS3 siRNA (1 nmol and 5 nmol) or control siRNA (5 nmol) treatment (n = 9 mice per treatment group). SA, sham air exposed; Eos, eosinophil; Mac, macrophage; Neu, neutrophil; Lym, lymphocyte. (B) Representative H&E-stained lung sections at 200× magnification. Quantitative analysis of epithelium thickness was performed blinded (n = 4 mice per treatment group). Values are shown as means ± SEMs. *Significant difference from control siRNA, p < 0.05; #significant difference from the SA group, p < 0.05.
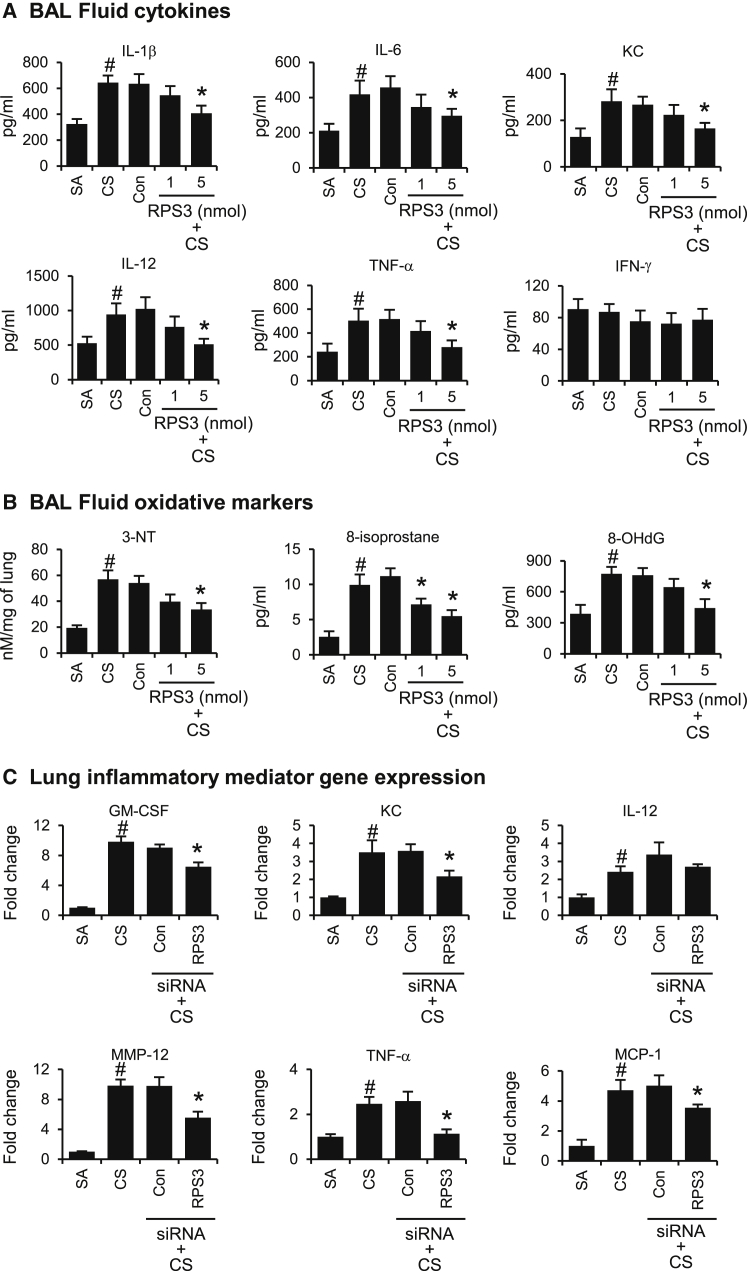
RPS3 Gene Silencing Attenuates CS-Induced Inflammatory Mediators and Oxidative Damage Markers
CS exposure significantly raised BAL fluid levels of inflammatory mediators, including IL-1β, IL-6, KC, IL-12, and TNF-α (Figure 5A), and oxidative damage markers such as 3-NT, 8-isoprostane, and 8-OHdG (Figure 5B). RPS3 siRNA dose-dependently suppressed BAL fluid levels of inflammatory mediators and oxidative damage markers, as compared with the non-targeting control siRNA (5 nmol) (Figures 5A and 5B). Based on protein analysis data from Figures 5A and 5B, primarily the higher dose of RPS3 siRNA (5 nmol) was able to significantly (p < 0.05) reduce CS-induced BAL fluid cytokine levels and oxidative damage markers. As such, we analyzed gene expression in lung tissues samples from mice treated with the higher dose of RPS3 siRNA at 5 nmol. Gene expression of inflammatory mediators, including GM-CSF, KC, MMP-12, TNF-α, and MCP-1 were also significantly suppressed by a higher dose of RPS3 siRNA (5nmol) in CS-challenged mouse lung, as compared with non-targeting control siRNA (5 nmol) (Figure 5C).
Figure 5.
Gene Silencing of RPS3 on BAL Fluid Cytokine and Oxidative Damage Marker Levels and Lung Tissue Inflammatory Gene Expression in CS-Induced Acute Lung Injury
(A) BAL fluid cytokine levels of IL-1β, IL-6, KC, IL-12, and TNF-α. SA, sham air exposed; CS, CS exposed. (B) BAL fluid oxidative damage markers 3-NT, 8-isoprostane, and 8-OHdG. (C) Lung tissue inflammatory gene expression modulated by RPS3 siRNA at 5 nmol. The relative quantity of target gene expression was normalized to that of β-actin as an internal control. Values are expressed as means of triplicate ± SEMs of 9 mice per treatment group. *Significant difference from control siRNA, p < 0.05; #significant difference from the SA group, p < 0.05.
RPS3 Gene Silencing Interferes with NF-κB Signaling Pathway
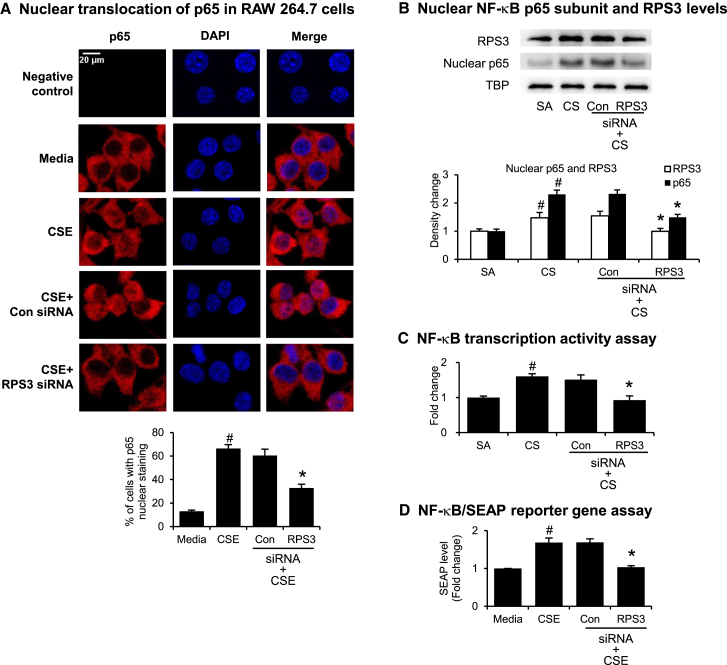
Using Alexa Fluor 633 to probe for NF-κB p65 subunit cellular distribution (Figure 6A, red signal), we observed that the p65 subunit was mainly located in the cytosol under resting conditions but that it began to translocate into the nucleus of RAW264.7 cells upon stimulation with CSE. Nuclei were stained with DAPI (Figure 6A, blue signal). RPS3 siRNA significantly inhibited CSE-induced nuclear accumulation of p65 subunit in RAW 264.7 cells, as shown by the drop in red fluorescence staining in the nucleus, as compared with the non-targeting control siRNA. The percentages of the cells in which the p65 staining overlapped with the DAPI nuclear staining were quantitated (Figure 6A). NF-κB p65 subunit and RPS3 nuclear accumulation were elevated in CS-challenged mouse lung tissues. Intratracheal administration of RPS3 siRNA (5 nmol) significantly reduced nuclear p65 and RPS3 levels, as compared with non-targeting control siRNA (5 nmol) (Figure 6B). CS challenge also increased nuclear p65 DNA-binding activity in lung tissue, which could be reversed by RPS3 siRNA treatment (Figure 6C). In an NF-κB secreted embryonic alkaline phosphatase (SEAP) reporter gene assay in vitro, RPS3 siRNA (5 nmol) was found to suppress CSE-induced SEAP protein levels in the supernatant of the NF-κB/SEAP reporter RAW 264.7 cells (Figure 6D).
Figure 6.
Effects of RPS3 Gene Silencing on NF-κB Translocation and Activity
(A) Nuclear translocation of p65 induced by CSE was captured using immunofluorescence staining in RAW 264.7 cells. Percentage of cells with p65 nuclear staining was quantified (n = 4 separate experiments). (B) Immunoblot of nuclear NF-κB subunit p65 and RPS3 accumulation. Mouse lung nuclear proteins were separated by 10% SDS-PAGE, and probed with anti-p65, anti-RPS3, or anti-TATA binding protein (TBP) mAbS. TBP was used as a nuclear protein loading control (n = 9 mice per treatment group). SA, sham air exposed. CS, CS exposed. (C) Nuclear p65 DNA-binding activity was determined using a TransAM p65 transcription factor ELISA kit (n = 9 mice per treatment group). (D) NF-κB reporter gene assay in NF-κB/SEAP reporter RAW 264.7 cells pre-treated with RPS3 siRNA and then stimulated with CSE. Results are expressed as fold change relative to media control. The SEAP assay was conducted in duplicate with three independent experiments. Values are shown as means of triplicate ± SEMs. *Significant difference from control siRNA, p < 0.05; #significant difference from the SA or control media group, p < 0.05.
Discussion
CS is the major risk factor for COPD, and CS exposure of mice allows for translational investigation of the pathophysiology of COPD and proof-of-concept drug study for the treatment of COPD.7, 19 CS activates epithelial cells and macrophages, leading to NF-κB pathway activation and inflammatory cell lung infiltration.2, 7 Infiltrated cells are attributable to the increased pro-inflammatory cytokine and chemokine levels, as well as the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In this study, we revealed for the first time that RPS3 protein levels were elevated in CSE-exposed mouse cell lines and CS-challenged mouse lungs, and intratracheal administration of RPS3 siRNA markedly attenuated airway inflammation and oxidative damage. We have also observed elevated p65 and RPS3 nuclear translocation in CS-challenged mouse lungs, and RPS3 gene silencing significantly inhibited their nuclear accumulation. In addition, RPS3 siRNA-mediated inhibition of NF-κB nuclear translocation and activation were captured by immunofluorescence staining and NF-κB/SEAP reporter gene assay.
NF-κB plays a central role in the airway inflammation in COPD.20 Bronchial biopsies of smokers revealed elevated p65 nuclear protein levels and nuclear translocation in bronchial epithelium, as compared with non-smokers. Increased NF-κB-DNA-binding activity was also observed in lung tissues of smokers, as compared with non-smokers.6 In addition, electrophoretic mobility shift assay (EMSA) has shown a significant increase in NF-κB nuclear translocation in the lung tissues from COPD patients.21 In experimental models, NF-κB activity was found upregulated in CS-challenged mouse lung and CSE-stimulated lung epithelial cells.9, 10, 11, 12 Li et al.22 reported that intratracheal NF-κB decoy oligodeoxynuleotides significantly ameliorated airway inflammation and prevented lung dysfunction in a CS-induced mouse COPD model, probably through the inhibition of NF-κB activity. In addition, the anti-inflammatory bioactive molecule andrographolide was found to mitigate the inflammatory response of human alveolar epithelial cells through inhibition of NF-κB activity via upregulation of miR-218.23
RPS3 has been shown to bind to the p65 subunit of the NF-κB complex via its KH domain, promote nuclear translocation of NF-κB complex, and enhance the binding affinity of the p65 subunit to downstream target gene promoters.14 In addition, inhibitory κB kinase β (IKKβ), an essential upstream kinase of the NF-κB pathway, has been shown to phosphorylate RPS3 at Ser209, crucial for subsequent RPS3 nuclear translocation.24 Overexpression of p65 did not enhance NF-κB activity when RPS3 was downregulated, indicating the obligatory role of RPS3 in activating NF-κB complex.14 Gene silencing of RPS3 has also shown to disrupt RPS3-p65 interaction and significantly attenuate cytokine production, T cell proliferation, and immunoglobulin κ light-chain gene expression in B cells.25
We observed significant elevation of BAL fluid total and differential cell counts, inflammatory cell infiltration into the peribronchiolar tissues, and substantial bronchial epithelium thickening in the CS-induced acute lung injury model. RPS3 knockdown markedly reduced total and neutrophil counts and, to a lesser extent, lymphocyte and eosinophil counts in BAL fluid. There was no significant change in macrophage counts, implying that RPS3 gene silencing may alter the functions of macrophages but was not able to significantly reduce the macrophage numbers. In the sputum and BAL fluid from COPD patients, the levels of TNF-α, IL-1β, and IL-6 are elevated, probably through activation of NF-κB-sensitive gene expression.26 TNF-α, as well as IL-8, enhances neutrophil trafficking into the lungs7. TNF-α also plays a predominant role in CS-induced emphysema.27 IL-1β can activate alveolar macrophages isolated from patients with COPD to secrete more inflammatory cytokines, chemokines, and MMP-9.26 IL-6 level is further elevated in the sputum and plasma in COPD patients with exacerbation.28 KC specifically recruits neutrophils to the airways, and neutrophils contribute to the release of proteases and additional KC in the lungs.29 Proteases, including MMP-8, MMP-9, and MMP-12 are elevated in the sputum of COPD patients, contributing to parenchymal destruction and emphysema in COPD.30, 31, 32 MCP-1 is a potent chemoattractant for monocytes, leading to the accumulation of macrophages in the lungs of COPD patients.33 CS induces GM-CSF secretion from airway epithelial cells, which triggers macrophage activation and accumulation, leading to persistent and progressive lung inflammation and fibrosis.7 In the present study, RPS3 gene silencing was able to suppress a wide spectrum of NF-κB-sensitive pro-inflammatory mediators in CSE-stimulated cells and CS-challenged mouse lungs by the disruption of NF-κB nuclear translocation and transactivation. RPS3 siRNA underscores its protective effects against CS-mediated acute lung injury.
Oxidative stress is heightened in COPD as a result of massive inhaled free radicals from CS as well as the release of ROS and RNS by the inflammatory and epithelial cells in the airway.34, 35 All these free radicals cause DNA, lipid, and protein damage, as shown by the production of 3-NT,36 8-isoprostane,37 and 8-OHdG,38 respectively, in BAL fluid or sputum from COPD patients. ROS and RNS are also involved in enhancing inflammation through the activation of NF-κB, which results in increased expression of pro-inflammatory mediators.38 We observed strong upregulation of all three oxidative damage markers in CS-induced acute lung injury model. RPS3 siRNA dose-dependently reduced all these markers in BAL fluid of CS-exposed mice, probably via inhibition of NF-κB pathway by mitigating iNOS expression and inflammatory cell infiltration.
Taken together, we report here for the first time that lung RPS3 level was significantly elevated in CS exposure, and lung RPS3 gene silencing protected against CS-induced airway inflammation, likely via disruption of the NF-κB pathway. Our findings strongly implicate RPS3 as a potential therapeutic target for the treatment of COPD.
Materials and Methods
In Vitro Characterization of RPS3 siRNA
Mouse RAW 264.7 macrophages and mouse LA-4 epithelial cells (American Type Culture Collection, Rockville, MD, USA) were maintained, respectively, in complete DMEM (Invitrogen, Carlsbad, CA, USA) and in Ham’s F12 medium (Biowest, Rue de la Caille, Nuaille, France) containing 1% MEM non-essential amino acids (Invitrogen). Human bronchial epithelial BEAS-2B cells were maintained in BEpiCM Bronchial Epithelial Cell Medium (ScienCell, San Diego, CA, USA). To silence RPS3 genes, cells were transfected with 100 nM RPS3 siRNA or non-targeting control siRNA for 6 hr at 37°C in OptiMEM (Invitrogen) containing Lipofectamine 2000 (Invitrogen). ON-TARGETplus siRNA and siSTABLE siRNA targeting mouse RPS3 mRNA, 5′-UCAUGUGAGCAUCGUGGAA-3′, and non-targeting control siRNA, 5′-UGGUUUACAUGUCGACUAA-3′, were purchased from Thermo Scientific (Waltham, MA, USA). Transfected cells were allowed to recover in complete DMEM or Ham’s F12 medium for 18, 42, or 66 hr before they were analyzed for RPS3 mRNA and protein expression. CSE was freshly prepared as previously described.8 To detect potential anti-inflammatory effects of RPS3 siRNA in vitro, transfected cells were stimulated with 4% CSE for 24 hr before analysis of pro-inflammatory gene and protein expression. To detect cytotoxicity of RPS3 siRNA, transfected cells were allowed to recover for 18, 42, or 66 hr before they were evaluated with an MTS assay (Promega, Madison, WI, USA).
Animals
Female BALB/c mice 6 to 8 weeks old were purchased from InVivos Pte. (Singapore). Mice were exposed to 3 sticks of 3R4F research cigarettes (Tobacco and Health Research Institute, University of Kentucky, Lexington, KY, USA) three times per day for 2 weeks (5 consecutive days/week). They were placed in a ventilated chamber filled with 4% CS or sham air delivered by peristaltic pumps (Masterflex L/S, Cole-Parmer Instrument Co., Niles, IL, USA) at a constant rate of 1 L/min. RPS3 siRNA (1 and 5 nmol) or non-targeting control siRNA (5 nmol) in 30 μL PBS was given once daily via intratracheal route on days 6 and 7 and 2 hr before CSE on days 8–11. Mice were euthanized by an i.p. injection of 300 μL of an anesthetic mixture (7.5 mg mL−1 ketamine plus 0.1 mg mL−1 medetomidine, obtained from the National University of Singapore Animal Holding Unit) on day 12 (Figure 3C). Animal experiments were performed following the guidelines of the Institutional Animal Care and Use Committee of the National University of Singapore under the protocol number 109/12.
BAL Fluid and Serum Analyses
BAL was performed as previously described,39 and BAL fluid total and differential cell counts were determined by flow cytometer (Fortessa, BD Biosciences, San Diego, CA, USA).40 BAL fluid levels of pro-inflammatory and oxidative markers were measured using ELISA (BD Biosciences and Cayman Chemical, Ann Arbor, MI, USA). Blood was collected by cardiac puncture immediately after last sham air or CS exposure for serum cotinine measurement using ELISA (Novus Biologicals, Littleton, CO, USA).
Lung Tissue and Histological Analyses
Total protein lysates and nuclear extracts (20 μg per lane) were separated by 10% SDS-PAGE and probed with anti-p65 mAb (Cell Signaling Technology, Beverly, MA, USA) or anti-RPS3 mAb (Abcam, Cambridge, MA, USA), and with anti-β-actin mAb (Cell Signaling Technology) or anti-TATA binding protein mAb (TBP; Abcam) as the internal control. Band intensity was quantitated using ImageJ software (NIH, Bethesda, MD, USA). Nuclear proteins were also analyzed for NF-κB p65 DNA-binding activity using the TransAM NF-κB p65 transcription factor assay kit (Active Motif, Carlsbad, CA, USA). Total mRNA was extracted using TRIzol reagent (Invitrogen). PCR amplifications were performed using primers listed in Table S2, and PCR products were analyzed by real-time qPCR (ABI 7500 Cycler; Applied Biosystems, Carlsbad, CA). The mRNA expression levels were normalized to the level of β-actin internal control. Lungs were fixed in 10% neutral formalin, paraffinized, cut into 5-μm sections, and stained with H&E for examining airway inflammation. Quantitative analyses of epithelium thickness were performed blinded as described.41 To visualize the lung distribution of RPS3-siRNA, mice were exposed to CS for 5 consecutive days, followed by intratracheal administration of 5 nmol Cy5-labeled RPS3 siRNA (red) (Thermo Scientific) on day 6. Mice were sacrificed 1 hr and 12 hr later, and lung sections were probed with anti-CD68 primary antibody followed by Alexa Fluor 488 fluorescent dye-conjugated secondary antibody (green) (Abcam). Nuclei were stained with DAPI (Sigma-Aldrich, St. Louis, MO, USA). Images were captured with an Olympus FluoView FV1000 confocal microscope (Olympus, Shinjuku, Tokyo, Japan).
NF-κB/SEAP Reporter Gene Assay
NF-κB/SEAP reporter RAW 264.7 cells (Novus Biologicals) were seeded in a 96-well plate at 5 × 104 cells in 100 μL and transfected with 100 nM RPS3 siRNA or non-targeting control siRNA for 6 hr. Transfected cells were then allowed to recover for 18 hr before stimulation with 4% CSE for another 24 hr. Quantitative measurements of SEAP protein levels were performed by using an NF-κB/SEAP assay kit (Novus Biologicals).
Immunofluorescence Staining
Raw 264.7 cells were seeded onto chamber slides and transfected with 100 nM RPS3 siRNA or non-targeting control siRNA. Transfected cells were then allowed to recover for 18 hr followed by stimulation with 4% CSE for 6 hr at 37°C. Cells were fixed, permeabilized, and probed with primary antibody targeted at NF-κB p65 (Cell Signaling) and Alexa Fluor 633 fluorescent dye-conjugated secondary antibody (Thermo Scientific), followed by DAPI (Sigma-Aldrich) staining. Images were captured with an Olympus FluoView FV1000 confocal microscope and quantified with ImageJ software (NIH). Experiments were repeated for four times, and percentages of p65 positive cells were quantified.
Statistical Analysis
Data are presented as mean ± SEM. Normal distribution was confirmed by the Kolmogorov-Smirnov test. A one-way ANOVA followed by Dunnett’s test was used to determine significant differences between treatment groups. Significant level was set at p < 0.05, as compared with non-targeting control siRNA.
Author Contributions
J.D., W.L., and W.S.F.W. conceived and designed the study. J.D., H.Y.P., W.S.D.T., and S.Z. participated in the acquisition of samples and data. J.D. and W.L. analyzed and interpreted the data. J.D., W.L., and W.S.F.W. wrote and revised the manuscript.
Conflicts of Interest
The authors have no competing financial interests.
Acknowledgments
This work was supported by CREATE grant R-184-000-269-592 from the National Research Foundation of Singapore (to W.S.F.W.) and NUHS bridging grant R-184-000-267-733 from the National University Health System, Singapore (to W.S.F.W.).
Footnotes
Supplemental Information includes two tables and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.05.027.
Supplemental Information
References
- 1.Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease (2015). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016.
- 4.Barnes P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013;12:543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- 5.Caramori G., Romagnoli M., Casolari P., Bellettato C., Casoni G., Boschetto P., Chung K.F., Barnes P.J., Adcock I.M., Ciaccia A. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax. 2003;58:348–351. doi: 10.1136/thorax.58.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Stefano A., Caramori G., Oates T., Capelli A., Lusuardi M., Gnemmi I., Ioli F., Chung K.F., Donner C.F., Barnes P.J., Adcock I.M. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur. Respir. J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- 7.Crotty Alexander L.E., Shin S., Hwang J.H. Inflammatory diseases of the lung induced by conventional cigarette smoke: a review. Chest. 2015;148:1307–1322. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 8.Guan S.P., Tee W., Ng D.S.W., Chan T.K., Peh H.Y., Ho W.E., Cheng C., Mak J.C., Wong W.S. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013;168:1707–1718. doi: 10.1111/bph.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solleti S.K., Simon D.M., Srisuma S., Arikan M.C., Bhattacharya S., Rangasamy T., Bijli K.M., Rahman A., Crossno J.T., Jr., Shapiro S.D., Mariani T.J. Airway epithelial cell PPARγ modulates cigarette smoke-induced chemokine expression and emphysema susceptibility in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309:L293–L304. doi: 10.1152/ajplung.00287.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmi S.P., Reddy A.T., Zhang Y., Sciurba F.C., Mallampalli R.K., Duncan S.R., Reddy R.C. Down-regulated peroxisome proliferator-activated receptor γ (PPARγ) in lung epithelial cells promotes a PPARγ agonist-reversible proinflammatory phenotype in chronic obstructive pulmonary disease (COPD) J. Biol. Chem. 2014;289:6383–6393. doi: 10.1074/jbc.M113.536805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Yang S.R., Valvo S., Yao H., Kode A., Rajendrasozhan S., Edirisinghe I., Caito S., Adenuga D., Henry R., Fromm G. IKK α causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am. J. Respir. Cell Mol. Biol. 2008;38:689–698. doi: 10.1165/rcmb.2007-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlahos R., Bozinovski S., Jones J.E., Powell J., Gras J., Lilja A., Hansen M.J., Gualano R.C., Irving L., Anderson G.P. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L931–L945. doi: 10.1152/ajplung.00201.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.D., Kim T.S., Joo Y.J., Shin H.S., Kim S.H., Jang C.Y., Lee C.E., Kim J. RpS3 translation is repressed by interaction with its own mRNA. J. Cell. Biochem. 2010;110:294–303. doi: 10.1002/jcb.22537. [DOI] [PubMed] [Google Scholar]
- 14.Wan F., Anderson D.E., Barnitz R.A., Snow A., Bidere N., Zheng L., Hegde V., Lam L.T., Staudt L.M., Levens D. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Wan F., Lenardo M.J. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J., Liao W., Peh H.Y., Chan T.K., Tan W.S.D., Li L., Yong A., Wong W.S.F. Ribosomal protein S3 gene silencing protects against experimental allergic asthma. Br. J. Pharmacol. 2017;174:540–552. doi: 10.1111/bph.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kole R., Krainer A.R., Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012;11:125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durcan N., Murphy C., Cryan S.A. Inhalable siRNA: potential as a therapeutic agent in the lungs. Mol. Pharm. 2008;5:559–566. doi: 10.1021/mp070048k. [DOI] [PubMed] [Google Scholar]
- 19.John G., Kohse K., Orasche J., Reda A., Schnelle-Kreis J., Zimmermann R., Schmid O., Eickelberg O., Yildirim A.Ö. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin. Sci. (Lond.) 2014;126:207–221. doi: 10.1042/CS20130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szulakowski P., Crowther A.J., Jiménez L.A., Donaldson K., Mayer R., Leonard T.B., MacNee W., Drost E.M. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;174:41–50. doi: 10.1164/rccm.200505-725OC. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.T., He B., Wang Y.Z., Wang J. Effects of intratracheal administration of nuclear factor-kappaB decoy oligodeoxynucleotides on long-term cigarette smoke-induced lung inflammation and pathology in mice. Respir. Res. 2009;10:79. doi: 10.1186/1465-9921-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y.J., Yu C.H., Li J.B., Wu X.Y. Andrographolide antagonizes cigarette smoke extract-induced inflammatory response and oxidative stress in human alveolar epithelial A549 cells through induction of microRNA-218. Exp. Lung Res. 2013;39:463–471. doi: 10.3109/01902148.2013.857443. [DOI] [PubMed] [Google Scholar]
- 24.Wan F., Weaver A., Gao X., Bern M., Hardwidge P.R., Lenardo M.J. IKKβ phosphorylation regulates RPS3 nuclear translocation and NF-κB function during Escherichia coli O157:H7 infection. Nat. Immunol. 2011;12:335–343. doi: 10.1038/ni.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wier E.M., Neighoff J., Sun X., Fu K., Wan F. Identification of an N-terminal truncation of the NF-κB p65 subunit that specifically modulates ribosomal protein S3-dependent NF-κB gene expression. J. Biol. Chem. 2012;287:43019–43029. doi: 10.1074/jbc.M112.388694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Culpitt S.V., Rogers D.F., Shah P., De Matos C., Russell R.E., Donnelly L.E., Barnes P.J. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- 27.Churg A., Wang R.D., Tai H., Wang X., Xie C., Wright J.L. Tumor necrosis factor-α drives 70% of cigarette smoke-induced emphysema in the mouse. Am. J. Respir. Crit. Care Med. 2004;170:492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 28.Bucchioni E., Kharitonov S.A., Allegra L., Barnes P.J. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir. Med. 2003;97:1299–1302. doi: 10.1016/j.rmed.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y., Li H., Bajrami B., Kwak H., Cao S., Liu P., Zhou J., Zhou Y., Zhu H., Ye K., Luo H.R. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. USA. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernooy J.H., Lindeman J.H., Jacobs J.A., Hanemaaijer R., Wouters E.F. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest. 2004;126:1802–1810. doi: 10.1378/chest.126.6.1802. [DOI] [PubMed] [Google Scholar]
- 31.Demedts I.K., Morel-Montero A., Lebecque S., Pacheco Y., Cataldo D., Joos G.F., Pauwels R.A., Brusselle G.G. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen C.A. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 34.van Eeden S.F., Sin D.D. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can. Respir. J. 2013;20:27–29. doi: 10.1155/2013/509130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H., Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide. 2011;25:138–144. doi: 10.1016/j.niox.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 37.Louhelainen N., Myllärniemi M., Rahman I., Kinnula V.L. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:585–603. doi: 10.2147/copd.s3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 39.Goh F.Y., Cook K.L., Upton N., Tao L., Lah L.C., Leung B.P., Wong W.S.F. Receptor-interacting protein 2 gene silencing attenuates allergic airway inflammation. J. Immunol. 2013;191:2691–2699. doi: 10.4049/jimmunol.1202416. [DOI] [PubMed] [Google Scholar]
- 40.Chan T.K., Loh X.Y., Peh H.Y., Tan W.N., Tan W.S., Li N., Tay I.J., Wong W.S., Engelward B.P. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J. Allergy Clin. Immunol. 2016;138:84–96.e1. doi: 10.1016/j.jaci.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Peh H.Y., Tan W.S.D., Chan T.K., Pow C.W., Foster P.S., Wong W.S.F. Vitamin E isoform γ-tocotrienol protects against emphysema in cigarette smoke-induced COPD. Free Radic. Biol. Med. 2017;110:332–344. doi: 10.1016/j.freeradbiomed.2017.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.