Abstract
In this manuscript we report the case of a 69-year-old female patient, who suffers from Parkinson's disease (PD) with a dilated Virchow-Robin space (dVRS) on the left anterior perforated substance.
During a magnetic resonance imaging examination, the presence of a dVRS was discovered on the left anterior perforated substance.
Subsequently, the patient has been subjected to further investigation of magnetic resonance imaging and diffusion tensor imaging (DTI). The DTI data of our PD patient showed increased peak frequency of left fractional anisotropy and decreases in the distribution of Mean Diffusivity(MD) with changes in the fiber density compared to the normal contralateral tract.
We hypothesize that the DTI changes are due to dVRS.
In the text a review of the recent literature on the presence of dVRSs, located in mono and bilateral seat, in patients with PD is reported, explaining its possible implications on disease progression, cognitive decline, and worsening of symptoms.
Keywords: Dilated Virchow-Robin spaces, Parkinson's disease, Magnetic resonance imaging (MRI), Diffusion tensor imaging (DTI)
Introduction
Virchow-Robin spaces (VRSs) are perivascular compartments surrounding small vessels within the central nervous system (CNS); they are filled by cerebrospinal fluid (CSF), with functional connections to subarachnoid space, and they contain vasoactive neuropeptides. Electron microscopic studies demonstrated that the pia mater separates perivascular and subpial (interstitial) spaces by encasing the wall of the vessels placed in the subarachnoid space [1].
VRSs were defined as ovoid, small, sharply delineated structures of CSF signal intensity on magnetic resonance (MR) brain studies that follow the orientation of the perforating vessels and run perpendicular to the brain surface, characterized by specific morphology and signal on MR studies [2]. VRSs have a regular contour, commonly seen bilaterally, and symmetrically or asymmetrically located and they often measure 5 mm or less. According to some authors, VRS are defined as dilated if their short axis is greater than 2 or 3 mm [2]. Topographically, there are 3 characteristic locations which are [2], [3]:
-
1.
Type I: along lenticulostriate arteries to the basal ganglia through the anterior perforated substance.
-
2.
Type II: they are in the path of the medullary perforating arteries when they enter in the gray matter heading toward the white matter.
-
3.
Type III: appears in the midbrain, mainly in the cerebral peduncles.
Occasionally, VRSs may have atypical features such as very large size with mass effect and consequent changes in cerebral spinal fluid (CSF) dynamics. Although several mechanisms have been hypothesized regarding their expansion, much remains to be confirmed especially in neurodegenerative diseases [4].
We report the case of a 69-year-old female patient, who suffers from Parkinson's disease (PD) with rigidity and bradykinesia, predominantly on the right limbs with a dVRS on the left anterior perforated substance discovered during a magnetic resonance imaging (MRI) examination. We contribute to the other case reports and series describing dVRSs and associations with various neurological entities and etiologies, including PD. Our case report does add to the relatively limited publications associating Parkinson symptoms and dilated perivascular spaces, potentially strengthening the correlation, although the overall evidence is limited. In the text, a review of the recent literature on the presence of dVRSs, located in mono and bilateral seat, in patients with PD is reported outlining its implications on disease progression, cognitive decline, and worsening of symptoms. The manuscript also describes and encourages the use of advanced imaging by diffusion tensor imaging (DTI) to better understand mass effect and its contributions to neurologic systems that is an exciting area of radiology. Diffusion tensor is a neuroimaging modality in the diagnosis and evaluation of numerous diseases of the nervous system. It allows the analysis of the bundles of nerve fibers that compose the white substance through the measurement of 2 parameters mean diffusivity (MD) which measures the global diffusion entity, calculated from the sum of the main diffusivities, and functional anisotropy fractional anisotropy (FA), which measures the preferential direction of the diffusion movement. Thanks to these parameters, it is possible to obtain information on the status of microscopic barriers (myelin, axonal membranes, and microtubules) that condition the diffusion of water in brain tissues and which can be affected by various pathological processes.
Case report
A 69-year-old woman developed PD with rigidity and bradykinesia, predominantly on the right limbs. The symptoms had appeared in full well-being with progressive trend. The patient did not have cerebrovascular risk factors. Family anamnesis showed that the mother was suffering from PD too. Non-motor symptoms reported by the patient were: constipation, anxiety, insomnia, and daytime sleepiness. The response to dopaminergic therapy has been positive.
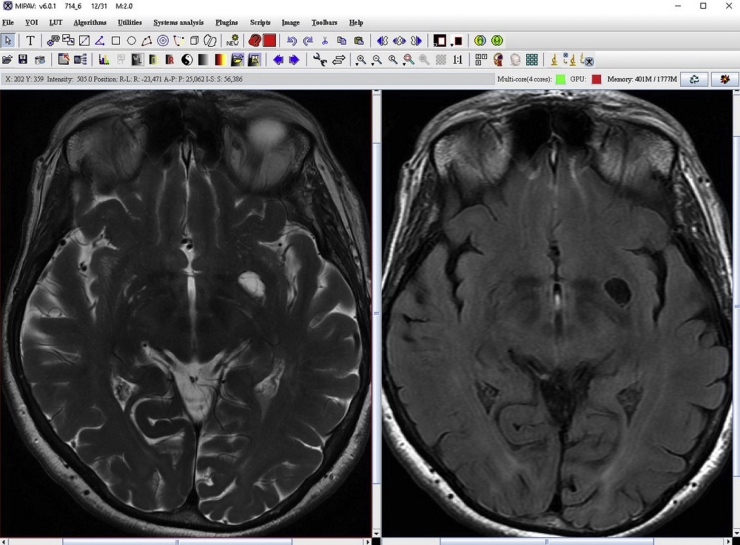
The MR study was performed on a 3 T high-field magnet Signa HDXT (General Electric, Milwaukee, Wisconsin), with head coil in following sequences: Transverse FRFSE T2 (TE 120, TR3440, EL 21, matrix 512 × 512, slice thickness 4 mm, spacing 0.4), FSE T2 FLAIR (TE 120, TR 9050, IT 2250, matrix 448 × 224, slice thickness 4 mm), and FSPGR Sagittal T1 (TE min full 2.8, prp time 650, FA 8, matrix 256 × 256, slice thickness 1.2 mm) 3D sequences. The MR data were analyzed by an expert radiologist with 20 years of experience, by a software analysis, the medical image processing analysis and visualization (MIPAV—Center for Information Technology, National Institutes of Health, Bethesda, Maryland). Through this semi-automatic post-processing applicative system, the left dVRS was visualized by SE T2, SE T1, and FLAIR and measured in T2 sequence (Fig. 1 MIPAV). Subsequently, a high-resolution DTI was performed.
Fig. 1.
Axial images on SE T2 and FLAIR sequences on the medical image processing analysis and visualization software analysis.
The data pre-processing consisted of the following steps: (1) the non-brain tissues from the head images were deleted by employing the brain extraction tool [5]; (2) the eddy current distortions and head motion artifacts were corrected and FA, MD, and RD maps were fitted with diffusion toolkit. Data were analyzed using FSL (www.fmrib.ox.ac.uk/fsl, Analysis Group, FMRIB, Oxford, UK) software, processed by a probabilistic tractography method based on multifiber model. Probabilistic tractography was performed using bilateral uncinate fasciculus by Johns Hopkins University Functional Magnetic Resonance Imaging of the Brain Software Library atlas (JHU FSL) atlas. All tractography maps were thresholded at 10% to reduce spurius connections. Left uncinate tractography map robust range intensity (min value 2nd percentile-max value 98th percentile) of FA, MD, and RD were 0.11-0.49, 6.7-1.3 × 10−3, and 5.0-1.15 × 10−3 as compared to 0.13-0.56, 0.1-0.3 × 10−5, and 0.1-0.1 × 10−5 on the normal contralateral side. Also left uncinate mapped FA, MD, and RD mean values respectively were: 0.30, 9.2 × 10−4, and 7.7 × 10−4, compared to 0.302, 8.6 × 10−4, and 7.0 × 10−4 on the right normal side. Comparisons were made between both sides for proportion of FA > 0.2 and distribution of FA. Histogram analysis showed increased peak frequency of FA and decreases in the distribution of MD. Left fiber density index was 0.49 (135.24/275) as compared to 0.7 (fibers/voxels = 157/222) for the normal contralateral tract (Table 1 and Fig. 2).
Table 1.
Histogram of FA distribution from 0.2 to 1 values for right (blue) and left (red) uncinated fasciculus.
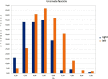 |
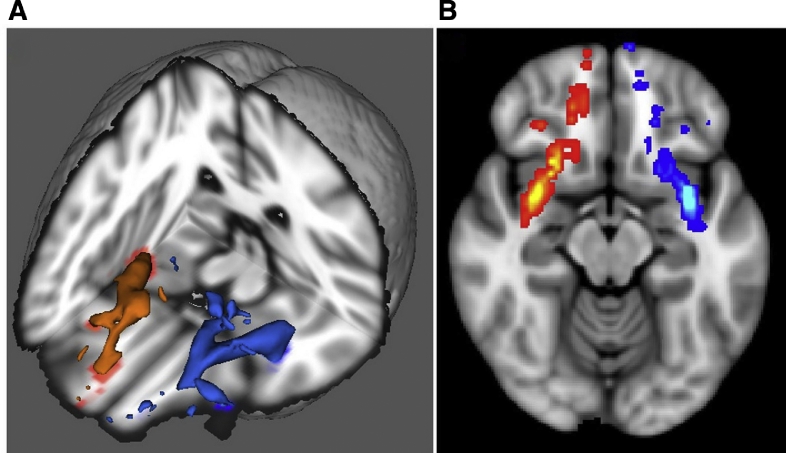
Fig. 2.
Left image shows 3D reconstructions of right (red) and left (blue) uncinate fasciculus. Right image shows diffusion image with probabilistic tractography of right (red) and left (blue) uncinate fasciculus.
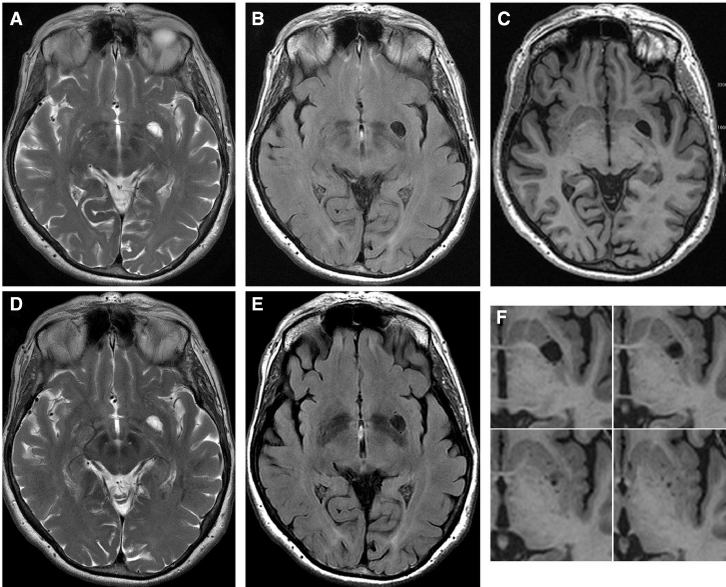
The T3 MRI brain study showed a dVRS type I to the left through the anterior perforated substance (Fig. 3 A,B and C), volumetrically unchanged on MRI follow-up studies (1 year later) (Fig. 3 D and E). The dVRS signal, in the various sequences, was compatible with that of the CSF. The dVRS, visible on 2 slices, on SE sequences, appeared as hyperintense on T2W images and hypointense on FLAIR and T1 sequence (Fig. 3). As confirmation of the perivascular origin of the dVRS cystic area, the SE T2 sequence highlights a small vascular structure inside (Fig. 3 A and D). The enlarged VRS measuring minor axis length of 13.4718 mm, area 384.728 mm2, and volume of 461.674 mm3. As appended after some time, we have found multiple thin brain VRSs around the main one (Fig. 3 F). Seemingly, the large VRS does not cause mass effect or alteration of the signal of the surrounding brain parenchyma.
Fig. 3.
The 3T MRI brain transverse SE T2, T1, and FLAIR W study shows left anterior perforated substance dilated Virchow-Robin space type I isointense to the cerebrospinal fluid (A, B and C). It is volumetrically unchanged on MRI follow-up studies (1 year later) (D and E). Note a small vascular structure in the dilated Virchow-Robin space (Fig. 1 A and B) and multiple thin smaller brain Virchow-Robin spaces around the main one (F).
Discussion
In contrast to what happened before the advent of MR studies, especially with high-field MRI, today it is very easy to identify, characterize, and diagnose the presence of VRS, observed along the course of intra-cerebral arteries, arterioles, venules, and veins. The VRSs have important functions such as: drainage of the CNS, immunology as an integral part of the blood-brain barrier; moreover, containing vasoactive neuropeptides that regulate blood pressure, heart rate, and microglia [2], [6], [7].
Typically, the visible VRS measures less than 2 mm, but they may expand and the causes of the dilatation are still unclear. Among various hypothesis, there are: segmental necrotizing angiitis of the arteries, conditions that change the permeability of their wall, brain atrophy with ex vacuum phenomenon, loss of myelin, disruption of the drainage route of interstitial fluid of the CNS, and diseases that cause fibrosis and obstruction of the VRS along the course of arteries and subsequent increased resistance to the fluid flow [6], [7], [8]. In the literature, it is reported that, their expansion (greater than 2 mm), is correlated with age, hypertension, senile dementia, and mild traumatic brain injury [7], [9]. In fact, Groeschel et al. claimed that the dVRSs are a normal phenomenon in elderly patients and should not be considered pathological findings [10]. The dVRS has been described as characteristic of many diseases: metabolic, genetic and vascular addition to multiple sclerosis, and PD [2], [4].
Analyzing whether there may be a correlation between dilated VRS and PD, we report the case of a patient suffering from PD, in which a MR 3T showed a significantly dilated left VRS (type I) located close to ipsilateral corticospinal tract and uncinate fascicle in the basal ganglia. The left uncinate fasciculus, adjacent to dVRS, showed increase in peak frequency of FA distribution, decreased MD, and thinning and compressions at probabilistic tractography. Further DTI study indicated, according to the literature, a possible correlation between the enlarged VRS and the starting symptoms of the patient.
Regarding the association between PD and dVRS, there are not many data in the literature.
In 2000, Laitinen et al., reported a single series of 40 patients with PD without mental deterioration scheduled for pallidotomy. All 40 patients had bilateral symptoms and signs, but 21 of them had predominance on the left side, and 18 on the right side. In that series, only 2 of 40 had unilateral dVRS in the striato-pallidal region, demonstrating the rarity of this finding. The authors think that striato-pallidal cysts develop from dVRS of the lenticulostriate vessels in the postero-ventral portions of striato-pallidal region. They are not pathognomonic for PD, but he claimed that they might play some role in lateralization of the clinical symptoms in this classically asymmetric condition [11]. In our patient, there is a single giant dVRS in the left anterior perforated substance and the symptoms are predominantly on the right limbs. In 2014, Mestre et al. presented 3 cases with atypical clinical features, which were affected by idiopathic PD [12]. In all cases, an isolated large VRS in the basal ganglia contralateral to the side of symptom onset was observed, similar to our patient. The authors propose that the atypical features could be associated with the mass effect of a significantly enlarged VRS, which would cause a dysfunction downstream from the presynaptic nigrostriatal dopaminergic system [12]. The authors conclude, stating that the seemingly rare finding of a single enlarged VRS in PD suggests that the underlying mechanism expanding VRS in these patients is likely independent from the neurodegenerative process ongoing in PD [12]. Further, Jamal et al. reported the case of a 52-year-old woman suffering from PD in whom brain MR incidentally found multiple cyst-like lesions as a giant VRS or “Swiss cheese” brain syndrome in the periventricular and juxta-cortical areas of hemispheres not associated with the patient's parkinsonism symptoms. In fact, the authors claimed that the giant VRS could be an incidental finding on brain imaging without any pertinent clinical manifestations [8]. Instead, in 2013, Mehta et al. reported the case of a 44-year-old woman with bilateral parkinsonism and a partial response to levodopa, associated with bilateral dVRS throughout the basal ganglia on brain MRI, and bilateral presynaptic deficit on DAT scan. The authors do not rule out the presence of coincidental Lewy body PD, but the incomplete response to levodopa suggests a contribution of the postsynaptic changes induced by dVRS [13].
In 2015, Fernandez et al., in a letter to the editor, described the case of a 55-year-old man with a 6 months long history of tremor in the left lower limb. When questioned directly, he reported fluctuating poor motor coordination with no hallucinations, falls, or any other symptoms. According to his family, his main symptom was apathy; they perceived no cognitive decline. Speech was slow and monotonous, with marked alterations in verbal fluency. Brain MRI and MR angiography study of the supraaortic trunks showed dVRS in the basal ganglia and both internal capsules, periventricular leukoencephalopathy predominantly in the fronto-parietal region bilaterally, and no alterations in the assessed cerebral vessels, or the circle of Willis. daTSCAN displayed intense dopaminergic deficit bilaterally with moderate non-specific uptake. The authors hypothesized that the Parkinson symptoms and altered higher functions in the patient may be linked to dVRS. The plausible explanation was that changes in the adjacent parenchyma secondary to bilateral dVRS may be involved because they would alter cortical-subcortical connections and involve the cortico-striatal fibers, thereby contributing to cognitive impairment and parkinsonism, respectively. From the DATSCAN daTSCAN findings, the authors cannot rule out other disorders such as PD or Lewy body dementia, which are associated with dVRS, as reported by Mehta et al. Thus, these dVRS could contribute to an earlier onset of the symptoms of these disorders [[14]]. In 2015, another author studied dVRS with MRI and positron emission tomography. Lee et al. presented a 64-year-old man, who developed parkinsonism and dementia [15].
MRI showed multiple round and septate cystic lesions, mainly in the right parietal, frontal, and temporal white matters. MR cerebral angiography showed no abnormalities. The positron emission tomography study showed a normal striatal dopamine transporter uptake, but reduced glucose metabolism in the right thalamus and right cerebral cortex. Therefore, the authors claimed that giant VRS in the high cerebral convexity are not always clinically silent but may cause progressive parkinsonism and dementia [15]. Lee et al., investigated the role of dVRS in the striatum on parkinsonism and dopamine transporter positron emission tomography (DAT-PET) findings in 11 patients [16]. From the results there was no significant correlation between dVRS and DAT-PET results [16]. The authors showed a significant correlation between dVRS and cognitive function tests. Thus, they claimed that dVRSs were present in various Parkinsonian disorders, the discrepancy between dVRS and DAT-PET, and the possible cognitive contribution of dVRS [16]. Moreover, in the literature, there are reported clinical studies of patients with dVRS investigated with DTI imaging, as we reported in this manuscript. Young et al. in 2014, reported the case of a 54-year-old female with peritoneal and breast carcinomas presented with headaches, memory loss, and confusion [17]. The patient had no signs or symptoms of motor weakness. The 1.5 T MRI brain study showed multiple brain metastases and edema distant from the motor cortices and corticospinal tracts. MRI brain study also revealed cystic dilatations in the left thalamus and ventral midbrain adjacent to the corticospinal tract. The DTI and tractography of the corticospinal tract showed displacement and compression of the corticospinal tract caused by mass effect from adjacent enlarged VRS. The corticospinal tract adjacent to the enlarged VRS showed significant changes in the proportion of FA > 0.8, distribution of FA, and distribution of MD (P < .001). In conclusion, the authors stated that enlarged VRS might induce asymptomatic diffusion tensor and tractography changes in the corticospinal tract through mass effect and compression.
These DTI data are very similar to those found in our patient, proving the mass effect on the brain parenchyma, due to the dVRS. To explain the relation between DTI changes, due to dVRS and PD symptoms we have to think that neurons in the nucleus basalis of Meynert provide major cholinergic input to the cerebral cortex via the cingulum, uncinate fasciculus, and the external capsule, so these white matter dysregulation might exacerbate cognitive and motor dysfunction in PD [18]. Other authors have studied verbal memory in 37 patients affected by PD matched with health control, with a combined DTI and functional MRI (fMRI) study that revealed significant dysfunctions in the verbal memory fMRI paradigm performance in PD patients compared to health control [19]. They have found evidence that supports impairments in verbal memory, FA reduction, and lower brain activation in PD patients [19]. These results revealed that deficits in the verbal memory in PD, rather than being solely accompanied by functional brain activation dysfunction, have a specific relationship with structural correlates related to white matter microstructural integrity [19]. Foo et al. in a 2016 review, provide the definition of the individual markers of small vessel disease [20]. They suggested the importance of each in affecting the pathophysiology of PD [20]. The author, as reported in the literature by Hatate et al. and van der Holst, claimed that the ischemic components of the pathological process of small vessel disease, white matter hyperintensities, chronic lacunar infarcts, perivascular or VRS, and microbleeds have been implicated in the manifestations of motor and non-motor symptoms in PD [21], [22]. In accordance with previous MRI studies (eg, reported by Mehta et al. in 2013), Foo et al. [20] reported that DTI studies showed correlations between changes in the corticospinal tracts and sensorimotor deficits and increased risk of dementia [23], [24].
The DTI data, of our PD patient, showed increased peak frequency of left FA and decreases in the distribution of MD with changes in the fiber density compared to the normal contralateral tract. Even if MRI morphologic brain study did not show evidence of compression signs due to enlarged VRS, we instead hypothesize that the DTI changes are due to dVRS. Because fMRI asymmetrical data does not entirely explain patient PD symptomatology, therefore larger sample of patients compared with healthy controls of MRI and fMRI studies will be made showing the relation between dVRS and PD symptoms.
Footnotes
Conflict of interest: The authors have declared that no competing interests exist.
Contributor Information
Renata Conforti, Email: renata.conorti@unicampania.it.
Angela Sardaro, Email: sardaroangela@libero.it.
Alberto Negro, Email: alberto.negro@hotmail.it.
Giuseppina Caiazzo, Email: gisysimona@hotmail.com.
Antonella Paccone, Email: anpaccone@gmail.com.
Rosita De Micco, Email: rosita.demicco@gmail.com.
Sossio Cirillo, Email: sossio.cirillo@unicampania.it.
Alessandro Tessitore, Email: alessandro.tessitore@unicampania.it.
References
- 1.Algin O, Conforti R, Saturnino PP, Ozmen E, Cirillo M, Di Costanzo A. Giant dilatations of Virchow-Robin spaces in the midbrain. MRI aspects and review of the literature. Neuroradiol J. 2012;25(4):415–422. doi: 10.1177/197140091202500404. First Published August 1. [DOI] [PubMed] [Google Scholar]
- 2.Conforti R, Cirillo M, Sardaro A, Caiazzo G, Negro A, Paccone A. Dilated perivascular spaces and fatigue: is there a link? Magnetic resonance retrospective 3Tesla study. Neuroradiology. 2016;58((September) 9):859–866. doi: 10.1007/s00234-016-1711-0. [DOI] [PubMed] [Google Scholar]
- 3.Wani NA, Mir F, Bhat IM, Gojwari T, Bhat S. Giant cystic Virchow-Robin spaces with adjacent white matter signal alteration. Turk Neurosurg. 2011;21(2):235–238. doi: 10.5137/1019-5149.JTN.2848-09.0. [DOI] [PubMed] [Google Scholar]
- 4.Conforti R, Cirillo M, Saturnino PP, Gallo A, Sacco R, Negro A. Dilated Virchow–Robin spaces and multiple sclerosis: 3T magnetic resonance study. Radiol Med. 2014;119((June) 6):408–414. doi: 10.1007/s11547-013-0357-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conforti R, Marrone V, Sardaro A, Di Maio N, Manzi F, Rossi M. Gli spazi perivascolari encefalici: revisione della letteratura sulle dilatazioni focali o diffuse. Recenti Prog Med. 2013;104(7):291–294. doi: 10.1701/1315.14562. [DOI] [PubMed] [Google Scholar]
- 7.Conforti R, Faella P, Marrone V, Iasiello F, Di Maio N, Rossi C. La dilatazione degli spazi di Virchow-Robin nel trauma cranico. Aspetti caratteristici in RM e revisione della letteratura. Recenti Prog Med. 2013;104(7):318–321. doi: 10.1701/1315.14568. [DOI] [PubMed] [Google Scholar]
- 8.Omidi SJ, Moghadam HN, Ghorbani A, Fatechi F. Giant Virchow-Robin spaces as an incidental finding in a patient with Parkinsonism. Arch Iran Med. 2014;17((August)8):587–588. [PubMed] [Google Scholar]
- 9.Fanous M, Media R. Perivascular spaces: normal and giant. Can J Neurol Sci. 2007;34:5–10. doi: 10.1017/s0317167100005722. [DOI] [PubMed] [Google Scholar]
- 10.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48((October) 10):745–754. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen LV, Chudy D, Tengvar M, Hariz MI, Bergenheim AT. Dilated perivascular spaces in the putamen and pallidum in patients with Parkinson's disease scheduled for pallidotomy: a comparison between; MRI findings and clinical symptoms and signs. Mov Disord. 2000;15(6):1139–1144. doi: 10.1002/1531-8257(200011)15:6<1139::aid-mds1012>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Mestre TA, Armstrong MJ, Walsh R, Al Dakheel A, Moro E, Stoessl AJ, Lang AE. Can isolated enlarged Virchow-Robin spaces influence the clinical manifestations of Parkinson's disease? Mov Disord Clin Pract. 2014;1((April) 1):67–69. doi: 10.1002/mdc3.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SH, Nichols FT, III, Espay AJ, Duker AP, Morgan JC, Sethi KD, FRCP (UK) Dilated Virchow– Robin Spaces and Parkinsonism. Mov Disord. 2013;28(5) doi: 10.1002/mds.25474. [DOI] [PubMed] [Google Scholar]
- 14.López Fernández M, Fraga Bau A, Volkmer García CM, Canneti Heredia B. Virchow-Robin spaces: a cause of Parkinsonism? Neurologia. 2016;31:493–494. doi: 10.1016/j.nrl.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Lyoo CH, Chung TS. Parkinsonism and dementia associated with giant Virchow-Robin spaces. J Mov Disord. 2015;8(2):106–107. doi: 10.14802/jmd.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee D, Hong IK, Ahn TB. Dilated Virchow-Robin space and dopamine transporter imaging in the striatum of patients with Parkinsonism. Can J Neurol Sci. 2015 doi: 10.1017/cjn.2015.43. [DOI] [PubMed] [Google Scholar]
- 17.Young RJ, Lee V, Peck KK, Sierra T, Zhang Z, Jacks LM, Holodny AI. Diffusion tensor imaging and tractography of the corticospinal tract in the presence of enlarged Virchow–Robin spaces. J Neuroimaging. 2014;24(1):79–82. doi: 10.1111/j.1552-6569.2011.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S. Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson's disease. Neurosci Lett. 2013;550:64–68. doi: 10.1016/j.neulet.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Lucas-Jiménez O, Díez-Cirarda M, Ojeda N, Peña J, Cabrera- Zubizarreta A, Ibarretxe-Bilbao N. Memory in Parkinson's disease: a combined DTI and fMRI study. J Parkinson's Dis. 2015;5:793–804. doi: 10.3233/JPD-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foo H, Kandiah N. The role of cerebrovascular disease in Parkinson's disease related cognitive impairment. J Parkinsons Dis Alzheimer Dis. 2016;3((October) 1):1–7. [Google Scholar]
- 21.Hatate J, Miwa K, Matsumoto M, Sasaki T, Yagita Y, Sakaguchi M. Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat Disord. 2016;26:29–34. doi: 10.1016/j.parkreldis.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 22.van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG. Cerebral small vessel disease and incident parkinsonism: the RUN DMC study. Neurology. 2015;85:1569–1577. doi: 10.1212/WNL.0000000000002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laundre BJ, Jellison BJ, Badie B, Alexander AL, Field AS 2005 diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol 26: 791–796. [PMC free article] [PubMed]
- 24.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]