Abstract
Background
Anagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor expected to improve the lipid profile as well as glycemic control. However, findings from large-scale prospective trials have not been obtained.
Methods
We performed a multicenter prospective trial in patients with type 2 diabetes receiving anagliptin to evaluate its effect on glycemic control and the lipid profile. A total of 95 patients received anagliptin at 200 mg twice daily. Markers of glucose and lipid metabolism were measured at baseline and after 12 and 24 weeks of administration, and the absolute changes and percent changes were determined.
Results
Both HbA1c and plasma glucose were significantly decreased by anagliptin therapy. Regarding the lipid profile, total cholesterol (TC) showed a significant decrease at 12 weeks, while TC, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were significantly decreased at 24 weeks. Multivariate analysis revealed that female sex was an independent predictor of greater reduction of TC, LDL-C, and HDL-C, while a baseline TC level ≥ 200 mg/dL predicted greater reduction of TC and a baseline HDL-C level ≥ 40 mg/dL predicted greater reduction of LDL-C and HDL-C.
Conclusions
This study suggested that anagliptin significantly reduced TC, LDL-C, and HDL-C levels, as well as improving glycemic control, particularly in female patients.
Keywords: Anagliptin, Dipeptidyl peptidase-4 inhibitor, Type 2 diabetes mellitus, Total cholesterol, Low-density lipoprotein cholesterol
Introduction
The increasing incidence of type 2 diabetes is a major problem [1, 2]. Cardiovascular disease is one of the chief causes of death associated with diabetes, and its frequency is 2-fold higher in patients with diabetes than in persons without diabetes [3]. The Japan Lipid Intervention Trial showed that the incidence of coronary heart disease (CHD) was 2.37 times higher in patients with diabetes than in non-diabetics [4].
Type 2 diabetes is associated with various abnormalities of lipid metabolism that increase the risk of cardiovascular disease, including hypertriacylglycerolemia, high levels of chylomicron remnants, elevation of small dense low-density lipoprotein (LDL), and a decrease in high-density lipoprotein (HDL) [5]. Insulin resistance underlies the development of type 2 diabetes. After the onset of insulin resistance, hepatic production of very-low-density lipoprotein (VLDL) increases due to an increase in free fatty acids (FFA) and hyperglycemia because of hyperinsulinemia. In addition, the activity of insulin-dependent lipoprotein lipase (LPL) decreases and the apoCIII content of VLDL increases. Furthermore, catabolism of VLDL is reduced, leading to high levels of both VLDL and lipoprotein remnants [6].
In Japanese patients with type 2 diabetes, the serum triglyceride (TG) level is an important predictor of CHD that shows comparable accuracy to LDL cholesterol (LDL-C). Because serum TG is not a leading predictor of CHD in patients with diabetes from Western countries, there may be a need to develop ethnic group-specific strategies for the prevention of diabetic macroangiopathy [7].
Anagliptin is a new selective inhibitor of dipeptidyl peptidase-4 (DPP-4), which is the enzyme responsible for inactivation of incretins, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide. Anagliptin improves glycemic control by stimulating insulin secretion through promotion of the activity of these incretins and suppression of excessive glucagon secretion [8]. Long-term treatment with anagliptin was reported to significantly decrease the LDL-C level (-13.9%) in patients with a baseline LDL-C ≥ 140 mg/dL [9].
This report indicated that treatment with anagliptin may improve lipid metabolism as well as glycemic control. Other DPP-4 inhibitors have also been reported to improve lipid parameters, and a meta-analysis showed that DPP-4 inhibitor treatment is associated with a significant reduction of total cholesterol (TC) [10]. We previously reported that both TC and non-HDL-C, but not LDL-C, were significantly reduced by administration of sitagliptin [11].
Recently, anagliptin was reported to improve both fasting and postprandial hyperlipidemia in men with type 2 diabetes [12], suggesting that anagliptin may be useful for treating hyperlipidemia as well as hyperglycemia. However, these findings were obtained by a small single-center study, suggesting the need for confirmation by a prospective multicenter trial.
Accordingly, we performed a multicenter trial to evaluate the effect of 12 and 24 weeks of anagliptin treatment on hemoglobin A1c (HbA1c) and lipid metabolism parameters in patients with type 2 diabetes complicated by dyslipidemia. We also investigated baseline demographic factors related to the clinical effects of anagliptin.
Materials and Methods
Patients
Patients with type 2 diabetes mellitus between the age of 20 - 85 years were enrolled in this study. Patients were eligible if their HbA1c levels were less than 9.0% without using insulin. Baseline characteristics of the subjects are shown in Table 1.
Table 1. Clinical and Laboratory Characteristics (Mean ± SD).
| Baseline | |
|---|---|
| N | 95 |
| Age (years) | 63.7 ± 11.7 |
| Age ≥ 60 | 67 (70.5%) |
| Sex, M/F | 59/36 |
| Weight (kg) | 66.3 ± 13.3 |
| BMI (kg/m2) | 25.4 ± 4.3 |
| TC (mg/dL) | 195.1 ± 41.6 |
| LDL-C (mg/dL) | 110.6 ± 37.1 |
| HDL-C (mg/dL) | 55.5 ± 13.8 |
| TG (mg/dL) | 148.1 ± 90.9 |
| Glucose (mg/dL) | 158.5 ± 41.0 |
| HbA1c (NGSP) (%) | 7.4 ± 0.7 |
| Neuropathy | 20 (21.0%) |
| Retinopathy | 15 (15.8%) |
| Nephropathy | 23 (24.2%) |
Study protocol
This study was carried out at nine medical institutions. Patients were treated with anagliptin at 200 mg twice daily for 24 weeks. After the study started, if they have been still inadequately controlled, it could be possible to dose up anagliptin to 400 mg twice daily. Throughout the study, any medications were not added and medications which patients took at entry had kept to be taken.
Patients provided written informed consent. The protocol of this study was approved by the Institutional Ethics Committee of Yokohama City University Medical Center (Yokohama, Japan) and it was performed in accordance with the Declaration of Helsinki. This study was registered with the UMIN Clinical Trials Registry System (UMIN 000012533).
Assessments
The main outcome measures were the changes in HbA1c levels and lipid profile.
At 0, 12 and 24 weeks, each patient’s body weight was measured and blood samples were collected to measure the levels of HbA1c, plasma glucose, serum TC, TG and HDL-C. The blood samples were taken from an antecubital vein at fasting in general. LDL-C level was estimated by Friedewald equation [13].
Continuous variables were presented as the mean ± SD while categorical variables are tabulated as frequencies and percentage. Data were compared by paired t-test between baseline, at week 12 and week 24. To identify factors associated with changes in TC, LDL-C, HDL-C and TG from baseline to week 12 and week 24, we performed univariate regression analysis. Then, we conducted multivariable regression analysis to identify independent predictors of the decrease in TC, LDL-C and HDL-C levels. P-value < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with SAS ver. 9.4 (SAS Institute Inc. Cary, NC).
Results
Baseline characteristics of the subjects are displayed in Table 1. Among 95 patients enrolled, 94 patients were followed for 12 weeks and 91 patients were followed for 24 weeks. Table 2 shows the baseline characteristics stratified by gender, revealing that there were no significant differences between male and female patients without in body weight, HDL-C and creatinine (Cr). When changes in glycemic control were investigated, anagliptin treatment significantly reduced HbA1c at 12 weeks and at 24 weeks. The blood glucose level also showed a significant decrease at 12 weeks and at 24 weeks. Body weight was significantly decreased at 12 weeks, but there was no significant change at 24 weeks (Table 3).
Table 2. Clinical and Laboratory Characteristics Stratified by Gender (Mean ± SD).
| Female | Male | P-value | |
|---|---|---|---|
| N | 36 | 59 | |
| Age (years) | 63.3 ± 12.5 | 64.0 ± 11.3 | 0.77 |
| Age ≥ 60 | 25 (69.4 %) | 42 (71.2 %) | |
| Weight (kg) | 62.9 ± 11.5 | 68.4 ± 13.9 | 0.049* |
| BMI (kg/m2) | 26.3 ± 4.3 | 24.9 ± 4.2 | 0.11 |
| TC (mg/dL) | 202.8 ± 43.7 | 190.1 ± 39.8 | 0.16 |
| LDL-C (mg/dL) | 114.5 ± 39.2 | 107.9 ± 35.7 | 0.42 |
| HDL-C (mg/dL) | 60.1 ±13.1 | 52.7 ± 13.5 | 0.010* |
| TG (mg/dL) | 140.9 ± 67.2 | 152.6 ± 103.3 | 0.55 |
| Glucose (mg/dL) | 148.8 ± 29.7 | 164.3 ± 45.7 | 0.076 |
| HbA1c (NGSP) (%) | 7.5 ± 0.6 | 7.3 ±0.7 | 0.39 |
| Cr (mg/dL) | 0.66 ± 0.12 | 0.86 ± 0.14 | < 0.001* |
| eGFR (mL/min/1.73m2) | 72.3 ± 14.6 | 72.3 ± 13.7 | 1.00 |
*P < 0.05.
Table 3. Amount of Change and % Change From Baseline to 12 Weeks and 24 Weeks.
| Baseline |
12 weeks |
24 weeks |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | % Change from baseline (mean (95% CL)) | Amount of change (mean ± SD) | P-value | Mean ± SD | % Change from baseline (mean (95% CL)) | Amount of change (mean ± SD) | P-value | |
| Weight (kg) | 66.3 ± 13.3 | 66.1 ± 13.5 | -1 (-1, -0.2) | -0.5 ± 1.8 | 0.016* | 66.7 ± 13.8 | 0 (-1, 0.4) | -0.2 ± 2.3 | 0.35 |
| TC (mg/dL) | 195.1 ± 41.6 | 188.2 ± 38.3 | -3 (-6, -0.1) | -7.6 ± 27.9 | 0.012* | 189.4 ± 36.5 | -3 (-5, -0.5) | -7.4 ± 22.1 | 0.0029* |
| LDL-C (mg/dL) | 110.6 ± 37.1 | 105.4 ± 34.2 | -2 (-7, 3.4) | -6.0 ± 28.7 | 0.06 | 104.7 ± 31.2 | -3 (-7, 0.5) | -7.3 ± 19.6 | 0.0014* |
| HDL-C (mg/dL) | 55.5 ± 13.8 | 55.2 ± 23.9 | 1 (-8, 11.3) | -0.4 ± 22.9 | 0.88 | 52.8 ± 14.0 | -4 (-6, -1.4) | -2.5 ± 7.0 | < 0.001* |
| TG (mg/dL) | 148.1 ± 90.9 | 156.4 ± 110.8 | 13 (2, 23.5) | 7.4 ± 80.5 | 0.38 | 171.6 ± 150.7 | 23 (3, 41.7) | 21.1 ± 129.9 | 0.13 |
| Glucose (mg/dL) | 158.5 ± 41.0 | 144.4 ± 37.8 | -6 (-11, -1.3) | -14.2 ± 40.9 | 0.0012* | 140.9 ± 36.9 | -7 (-13, -0.9) | -17.9 ± 52.0 | 0.0015* |
| HbA1c (%) | 7.4 ± 0.7 | 7.0 ± 0.8 | -5 (-6, -3) | -0.3 ± 0.6 | < 0.001* | 7.0 ± 0.9 | -5 (-7, -2.8) | -0.4 ± 0.8 | < 0.001* |
*P < 0.05.
Regarding the changes in lipid parameters with anagliptin treatment, TC was significantly decreased at 12 weeks and at 24 weeks. In contrast, LDL-C and HDL-C levels did not show significant changes at 12 weeks. However, LDL-C was significantly decreased at 24 weeks by -3% and HDL-C also showed a significant reduction. TG did not change significantly from baseline at either 12 weeks or 24 weeks (Table 3).
Subgroup analysis
We investigated the effects of anagliptin in subgroups of patients stratified according to age (< 60 vs. ≥ 60), gender, body mass index (BMI) (< 25 vs. ≥ 25 kg/m2), baseline TC (< 200 vs. ≥ 200 mg/dL), baseline LDL-C (< 120 vs. ≥ 120 mg/dL), baseline HDL-C (< 40 vs. ≥ 40 mg/dL), baseline TG (< 150 vs. ≥ 150 mg/dL), lipid-lowering therapy (yes vs. no), and statin therapy (yes vs. no) (Table 4).
Table 4. % Change From Baseline at 24 Weeks With Different Background.
| N | TC | P-value | LDL-C | P-value | HDL-C | P-value | TG | P-value | Glucose | P-value | HbA1c | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||||
| < 60 | 26 | -2.9 ± 7.3 | 0.93 | -5.5 ± 13.4 | 0.52 | -6.3 ± 11.0 | 0.21 | 55.8 ± 134.3 | 0.1 | -2.8 ± 33.7 | 0.45 | -0.3 ± 10.3 | 0.01* |
| ≥ 60 | 65 | -2.7 ± 11.3 | -2.9 ± 19.0 | -2.9 ± 12.6 | 9.1 ± 62.9 | -8.5 ± 26.2 | -6.5 ± 8.7 | ||||||
| Sex | |||||||||||||
| Female | 33 | -5.2 ± 10.8 | 0.09 | -7.6 ± 17.4 | 0.083 | -3.1 ± 12.3 | 0.65 | 11.2 ± 64.9 | 0.32 | -3.0 ± 22.5 | 0.30 | -7.8 ± 8.0 | 0.013* |
| Male | 58 | -1.2 ± 9.8 | -0.6 ± 17.6 | -4.3 ± 12.3 | 29.2 ± 103.4 | -9.0 ± 31.2 | -3.0 ± 10.0 | ||||||
| BMI | |||||||||||||
| <25 | 44 | -3.5 ± 10.7 | 0.53 | -4.4 ± 15.8 | 0.63 | -3.9 ± 11.3 | 0.97 | 13.6 ± 65.1 | 0.36 | -8.1 ± 24.0 | 0.69 | -5.6 ± 7.6 | 0.43 |
| ≥25 kg/m2 | 47 | -2.1 ± 10.0 | -2.4 ± 20.0 | -3.9 ± 13.1 | 31.2 ± 110.7 | -5.7 ± 32.3 | -4.0 ± 11.1 | ||||||
| Baseline | |||||||||||||
| TC (< 200 mg/dL) | 48 | 0.7 ± 9.7 | < 0.001* | 2.6 ± 18.2 | < 0.001* | -1.1 ± 12.8 | 0.017* | 15.0 ± 66.9 | 0.43 | -8.1 ± 23.5 | 0.68 | -6.1 ± 9.2 | 0.20 |
| TC (≥ 200 mg/dL) | 39 | -7.1 ± 9.5 | -12.0 ± 13.3 | -7.3 ± 10.6 | 32.1 ± 118.6 | -5.4 ± 34.1 | -3.5 ± 9.7 | ||||||
| LDL-C (< 120 mg/dL) | 49 | -0.8 ± 10.0 | 0.02* | 1.4 ± 18.6 | 0.001* | -2.5 ± 12.7 | 0.17 | 10.3 ± 51.7 | 0.18 | -5.4 ± 30.4 | 0.70 | -5.0 ± 10.2 | 0.81 |
| LDL-C (≥ 120 mg/dL) | 35 | -6.1 ± 10.1 | -10.7 ± 13.7 | -6.1 ± 11.2 | 42.3 ± 130.9 | -7.9 ± 27.0 | -4.5 ± 8.5 | ||||||
| HDL-C (< 40 mg/dL) | 11 | 3.7 ± 11.1 | 0.056 | 17.2 ± 26.4 | 0.063 | 6.3 ± 11.5 | 0.008* | 5.5 ± 75.7 | 0.47 | 1.4 ± 41.1 | 0.48 | -5.5 ± 16.7 | 0.87 |
| HDL-C(≥ 40 mg/dL) | 80 | -3.7 ± 9.9 | -5.5 ± 15.5 | -5.3 ± 11.7 | 24.7 ± 93.1 | -8.0 ± 26.4 | -4.7 ± 8.3 | ||||||
| TG (< 150 mg/dL) | 55 | -2.5 ± 10.1 | 0.74 | -5.3 ± 14.3 | 0.3 | -5.4 ± 13.0 | 0.18 | 36.3 ± 106.6 | 0.04* | -9.3 ± 27.9 | 0.32 | -5.6 ± 10.0 | 0.25 |
| TG (≥ 150 mg/dL) | 35 | -3.3 ± 11.0 | -0.3 ± 22.7 | -2.0 ± 10.7 | 1.0 ± 54.2 | -3.0 ± 29.6 | -3.2 ± 8.7 | ||||||
| Lipid treatment | |||||||||||||
| No | 44 | -4.6 ± 10.2 | 0.13 | -7.1 ± 15.2 | 0.084 | -4.0 ± 11.8 | 0.92 | 25.9 ± 105.5 | 0.74 | -12.1 ± 24.6 | 0.084 | -5.2 ± 10.8 | 0.69 |
| Yes | 47 | -1.2 ± 10.3 | -0.2 ± 19.4 | -3.8 ± 12.7 | 19.4 ± 76.1 | -1.8 ± 31.1 | -4.4 ± 8.3 | ||||||
| Statin | |||||||||||||
| No | 48 | -3.2 ± 10.7 | 0.71 | -6.6 ± 15.3 | 0.12 | -3.8 ± 11.8 | 0.95 | 30.4 ± 105.9 | 0.38 | -9.7 ± 30.2 | 0.31 | -4.9 ± 11.5 | 0.88 |
| Yes | 43 | -2.3 ± 10.0 | -0.3 ± 19.7 | -4.0 ± 12.9 | 13.7 ± 71.1 | -3.6 ± 26.3 | -4.6 ± 6.9 |
*P < 0.05.
Anagliptin treatment achieved significantly greater reduction of HbA1c in patients over 60 years old than in those under 60 years old, as well as in female patients compared with male patients.
Regarding lipid parameters, anagliptin treatment achieved significantly greater reduction of TC at 24 weeks in patients with a baseline TC level ≥ 200 mg/dL than in those with a baseline TC < 200 mg/dL, and in patients with a baseline LDL-C level ≥ 120 mg/dL than in those with a baseline LDL-C < 120 mg/dL. Treatment with anagliptin also achieved significantly greater reduction of LDL-C at 24 weeks in patients with a baseline TC ≥ 200 mg/dL than in those with a baseline TC < 200 mg/dL, and in patients with a baseline LDL-C ≥ 120 mg/dL than in those with a baseline LDL-C < 120 mg/dL. The percent change in HDL-C from baseline to 24 weeks was significantly larger in the subgroup with a baseline TC ≥ 200 mg/dL than in the subgroup with a baseline TC < 200 mg/dL, as well as in the subgroup with a baseline HDL-C ≥ 40 mg/dL than in the subgroup with a baseline HDL-C < 40 mg/dL. The percent change in TG from baseline to 24 weeks was also significantly larger in the subgroup with a baseline TG < 150 mg/dL than in the subgroup with a baseline TG ≥ 150 mg/dL. However, there were no obvious differences between the subgroups with or without medications for dyslipidemia.
Correlation between the percent change in HbA1c and the changes in TC, LDL-C, or HDL-C
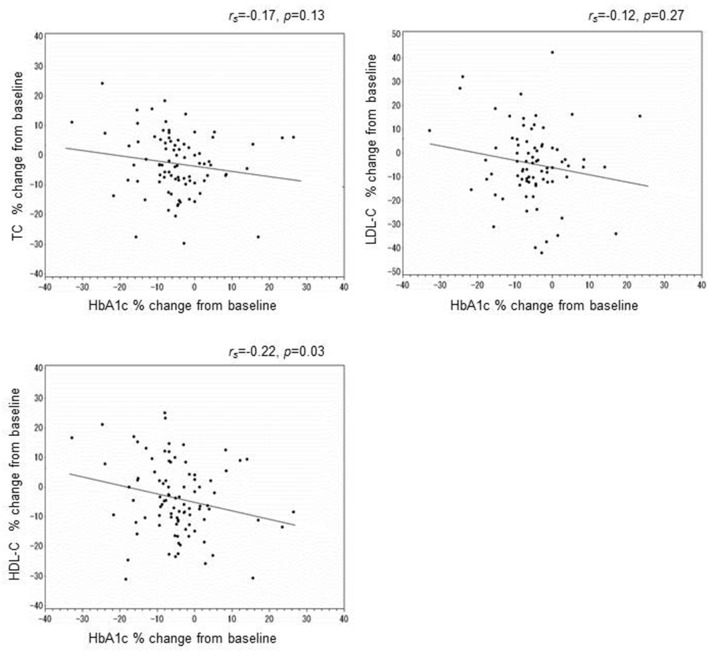
Next, to elucidate whether improvement in blood glucose affected lipid parameters, we investigated the association between changes in HbA1c and changes in the lipid profile. However, there were no associations between the change in HbA1c and the changes in TC, HDL-C, or LDL-C at 24 weeks (Fig. 1).
Figure 1.
Correlation between the percent change in HbA1c and that in TC, LDL-C, or HDL-C at 24 weeks.
Results of univariate and multivariate analysis
Table 5 shows the results of univariate and multivariate analyses that were performed to identify independent predictors of reduction of the TC, HDL-C, and LDL-C levels by anagliptin. After adjustment for age, BMI, and sex, univariate analysis showed that a baseline TC level ≥ 200 mg/dL, baseline HDL-C level ≥ 40 mg/dL, and baseline LDL-C level ≥ 120 mg/dL were independent predictors of larger reduction of TC and LDL-C at 24 weeks. In addition, a baseline TC level ≥ 200 mg/dL and baseline HDL-C level ≥ 40 mg/dL were independent predictors of larger reduction of HDL-C at 24 weeks. However, multivariate analysis showed that female sex and a baseline TC level ≥ 200 mg/dL were independent predictors of greater TC reduction, while female sex and a baseline HDL-C level ≥ 40 mg/dL were independent predictors of greater LDL-C and HDL-C reduction.
Table 5. Univariate and Multivariate Analysis.
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | ||||
| % change in TC at 24 weeks | |||||||
| Age | ≥ 60 | 0.2 | (-4.8, 5.2) | 0.94 | -1.7 | (-6.4, 3.0) | 0.47 |
| Sex | Male | 4.0 | (-0.4, 8.4) | 0.075 | 3.9 | (-0.1, 8.0) | 0.055 |
| BMI | ≥ 25 kg/m2 | 1.4 | (-2.9, 5.8) | 0.52 | 2.4 | (-1.9, 6.6) | 0.27 |
| Baseline TC | ≥ 200 mg/dL | -7.9 | (-11.9, -3.8) | < 0.001* | -10.1 | (-15.7, -4.6) | < 0.001* |
| Baseline LDL-C | ≥ 120 mg/dL | -5.3 | (-9.7, -1.0) | 0.016* | 1.8 | (-3.8, 7.4) | 0.53 |
| Baseline HDL-C | ≥ 40 mg/dL | -7.4 | (-13.8, -1.1) | 0.021* | -3.7 | (-10.5, 3.2) | 0.29 |
| Baseline TG | ≥ 150 mg/dL | -0.8 | (-5.4, 3.7) | 0.73 | |||
| Lipid therapy | Yes | 3.4 | (-0.9, 7.7) | 0.12 | |||
| Statin | Yes | 0.8 | (-3.5, 5.2) | 0.71 | |||
| % change in LDL-C at 24 weeks | |||||||
| Age | ≥ 60 | 2.6 | (-6.5, 11.7) | 0.58 | -1.2 | (-9.3, 7.0) | 0.78 |
| Sex | Male | 7.0 | (-0.7, 14.8) | 0.075 | 7.2 | (0.3, 14.1) | 0.04* |
| BMI | ≥ 25 kg/m2 | 2.0 | (-5.8, 9.8) | 0.62 | 3.1 | (-4.1, 10.3) | 0.40 |
| Baseline TC | ≥ 200 mg/dL | -14.6 | (-21.8, -7.4) | < 0.001* | -9.3 | (-19.2, 0.6) | 0.065 |
| Baseline LDL-C | ≥ 120 mg/dL | -12.1 | (-19.5, -4.6) | 0.002* | -3.9 | (-13.7, 5.9) | 0.44 |
| Baseline HDL-C | ≥ 40 mg/dL | -22.7 | (-35.5, -10.0) | < 0.001* | -16.7 | (-29.0, -4.4) | 0.008* |
| Baseline TG | ≥ 150 mg/dL | 5.0 | (-3.1, 13.0) | 0.23 | |||
| Lipid therapy | Yes | 6.9 | (-0.8, 14.5) | 0.078 | |||
| Statin | Yes | 6.2 | (-1.4, 13.9) | 0.11 | |||
| % change in HDL-C at 24 weeks | |||||||
| Age | ≥ 60 | 3.4 | (-2.1, 8.9) | 0.22 | -1.2 | (-9.3, 7.0) | 0.78 |
| Sex | Male | -1.2 | (-6.4, 4.0) | 0.65 | 7.2 | (0.3, 14.1) | 0.04* |
| BMI | ≥ 25 kg/m2 | -0.1 | (-5.1, 4.9) | 0.97 | 3.1 | (-4.1, 10.3) | 0.40 |
| Baseline TC | ≥ 200 mg/dL | -6.1 | (-11.1, -1.2) | 0.015* | -9.3 | (-19.2, 0.6) | 0.065 |
| Baseline LDL-C | ≥ 120 mg/dL | -3.7 | (-8.9, 1.5) | 0.17 | -3.9 | (-13.7, 5.9) | 0.44 |
| Baseline HDL-C | ≥ 40 mg/dL | -11.6 | (-18.9, -4.3) | 0.002* | -16.7 | (-29, -4.4) | 0.008* |
| Baseline TG | ≥ 150 mg/dL | 3.4 | (-1.7, 8.5) | 0.19 | |||
| Lipid therapy | Yes | 0.3 | (-4.7, 5.3) | 0.92 | |||
| Statin | Yes | -0.2 | (-5.2, 4.8) | 0.95 | |||
*P < 0.05.
Discussion
This study evaluated the effects of anagliptin on glycemic control and the lipid profile in patients with inadequately controlled type 2 diabetes. The main findings were that administration of anagliptin (200 mg) led to a significant decrease in HbA1c and glucose at 12 and 24 weeks, as well as significant reduction of TC at 12 and 24 weeks, along with a significant reduction of HDL-C and LDL-C at 24 weeks.
Improvement in the lipid profile by DPP-4 inhibitors was reported recently. A meta-analysis that examined the lipid-lowering effect of DPP-4 inhibitors revealed a decrease in TC [10]. However, consensus has not yet been achieved about the changes in lipids after administration of these drugs [10-12]. In the case of anagliptin, a significant LDL-C-lowering effect of long-term administration has been confirmed [9].
In that report, the change in LDL-C at 24 weeks was 4.7±0.7%, while it was 3% in the present study, the reduction of LDL-C was slightly smaller in our study. The difference between the two studies may have been related to different baseline LDL-C levels. Baseline LDL-C was lower in this study than in the previous study, and this may have contributed to the smaller percent change in LDL-C in the present study.
The previous study also showed that a high baseline LDL-C level was associated with a larger decrease in LDL-C. We obtained comparable result in subgroup analysis, with a larger decrease in the subgroup with a baseline LDL-C level ≥ 120 mg/dL.
Next, there was no correlation between the percent change in HbA1c and the percent change in LDL-C, which showed that improvement in LDL-C was not related to improved glycemic control. These findings suggest that anagliptin may have a mechanism that is unique among DPP-4 inhibitors for reducing LDL-C levels.
A previous study suggested that the TC-lowering action of anagliptin is related to inhibition of hepatic TC synthesis [14]. It was also recently reported that anagliptin acts on sterol regulatory element-binding protein 2 (SREBP-2), a regulator of cholesterol metabolism in hepatocytes [15]. SREBP-2 regulates both the LDL receptor and the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase to control the cholesterol concentration in hepatocytes. Administration of anagliptin to LDL receptor-deficient mice suppressed SREBP-2 expression and hepatic lipid synthesis. These findings suggested that anagliptin had a unique mechanism for improving the lipid profile. Furthermore, a recent study reported that anagliptin treatment significantly improved serum apoB-100 levels and suggested that the LDL-lowering effect of anagliptin is mediated, at least in part, through the suppression of apoB-100 synthesis [16].
In this study, multivariate analysis demonstrated that female sex was an independent predictor of LDL-C reduction, apparently due to the difference in the number of LDL receptors between males and females. LDL receptors are decreased in postmenopausal women, and this is the trigger for postmenopausal elevation of LDL-C [17]. Since the average age of menopause is about 50 years in Japan, 83.3% of the female patients in this study were estimated to be postmenopausal (data not shown). Accordingly, LDL receptor expression may have been decreased in over 80% of the female patients, which could be associated with the differing effect of anagliptin on LDL-C levels in men and women. Since GLP-1 analogues are known to upregulate LDL-receptor expression [18], the reduction of LDL-C by anagliptin could also be related to elevation of LDL-receptor expression.
Regarding other parameters, a significant change in TG was not observed at 24 weeks, although subgroup analysis showed a significant increase in the baseline TG < 150 mg/dL subgroup. TG-lowering effect of anagliptin and other DPP-4 inhibitors have already been reported [9, 19], possibly due to reduced appetite and suppression of the production of TG-rich lipoproteins and ApoB48 [20]. However, the subgroup analysis performed in this study showed a significant increase in TG in the baseline TG < 150 mg/dL group, unlike the previous study. Further investigation is required to reveal the cause of this discrepancy.
HDL-C was also significantly lower at 24 weeks. Subgroup analysis revealed that HDL-C decreased in the baseline HDL-C ≥ 40 mg/dL subgroup, while it tended to increase in the baseline HDL-C < 40 mg/dL group. During long-term administration, HDL-C was decreased at 24 weeks and subsequently tended to increase in the baseline HDL-C < 40 mg/dL subgroup. Although anagliptin may improve HDL-C in patients with low HDL-C levels, it may possibly have an unfavorable effect in patients with high HDL-C levels, but this could not be clarified by the present study.
The incidence of arteriosclerotic diseases is 2 to 3 times higher in diabetic patients compared with non-diabetic persons [21], and the incidence of ischemic heart disease increases further if diabetes is complicated by dyslipidemia [22]. Various large-scale intervention studies have obtained evidence that improvement of dyslipidemia reduces cardiovascular events. For example, the Collaborative Atorvastatin Diabetes Study (CARDS) reported the reduction of major coronary events by 37% and reduction of death by 27% [23]. The current LDL-C target value is < 120 mg/dL [24]. Baseline LDL-C was 110.6 ± 31.7 mg/dL in the present study, which is within the target range, but LDL-C improved further to 105.4 ± 34 mg/dL after administration of anagliptin. Management of dyslipidemia is very important for the prevention of cardiovascular disease. Since anagliptin significantly improves LDL-C as well as glycemic control, it is considered to be useful for patients with diabetes and dyslipidemia. In particular, administration to postmenopausal women could be beneficial. However, our findings were not in agreement with previous reports about an increase in TG and a decrease in HDL-C with anagliptin therapy [9], and further investigation seems to be required.
Acknowledgments
This work was conducted independently; no company or institution supported it financially. We thank all the physicians who participated in this study. We also thank Mrs. Yamagiwa, Morimoto, and Seki for secretarial assistance.
Disclosure
Terauchi Y received honoraria for lectures from MSD; Ono Pharmaceutical Co. Ltd.; Boehringer Ingelheim GmbH; Takeda Pharmaceutical Company Ltd.; Tanabe-Mitsubishi Pharma; Daiichi-Sankyo Company Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly and Company; Sanofi; DaiNippon-Sumitomo; Shionogi & Co., Ltd.; Bayer Yakuhin Ltd.; Astellas Pharma Inc.; and Astra Zeneca and obtained research support from MSD; Ono Pharmaceutical Co. Ltd.; Boehringer Ingelheim GmbH; Takeda Pharmaceutical Company Ltd.; Tanabe-Mitsubishi Pharma; Daiichi-Sankyo Company Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly and Company; Sanofi; Astellas Pharma Inc.; and Astra Zeneca. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH. et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y. et al. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J. 2002;66(12):1087–1095. doi: 10.1253/circj.66.1087. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg HN, Huang LS. The insulin resistance syndrome: impact on lipoprotein metabolism and atherothrombosis. J Cardiovasc Risk. 2000;7(5):325–331. doi: 10.1177/204748730000700505. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino G, Kazumi T, Iwai M, Matsuba K, Iwatani I, Matsushita M, Kasama T. et al. Recommendation for strict control of plasma triglyceride in diabetic subjects. Diabetes Care. 1988;11(10):794–795. doi: 10.2337/diacare.11.10.794. [DOI] [PubMed] [Google Scholar]
- 7.Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S. et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS) J Clin Endocrinol Metab. 2011;96(11):3448–3456. doi: 10.1210/jc.2011-0622. [DOI] [PubMed] [Google Scholar]
- 8.Kaku K. Efficacy and safety of anagliptin add-on therapy in Japanese patients with type 2 diabetes-Placebo-controlled, double-blind, parallel-group study with an open-label, long-term extension. Jpn Pharmacol Ther. 2012;40(9):745–770. [Google Scholar]
- 9.Kaku K. Effects of anagliptin on serum lipids in Japanese patients with type2 diabetes -A pooled analysis of long-term therapy with anagliptin. Jpn Pharmacol Ther. 2012;40(9):771–784. [Google Scholar]
- 10.Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29(1):14–25. doi: 10.1007/s12325-011-0088-z. [DOI] [PubMed] [Google Scholar]
- 11.Shigematsu E, Yamakawa T, Kadonosono K, Terauchi Y. Effect of sitagliptin on lipid profile in patients with type 2 diabetes mellitus. J Clin Med Res. 2014;6(5):327–335. doi: 10.14740/jocmr1889w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakuda H, Kobayashi J, Kakuda M, Yamakawa J, Takekoshi N. The effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetes. Endocrine. 2015;48(3):1005–1009. doi: 10.1007/s12020-014-0376-x. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 14.Aoki K, Ijima T, Kamiyama H, Kamiko K, Terauchi Y. Anagliptin decreases serum lathosterol level in patients with type 2 diabetes: a pilot study. Expert Opin Pharmacother. 2015;16(12):1749–1754. doi: 10.1517/14656566.2015.1057120. [DOI] [PubMed] [Google Scholar]
- 15.Yano W, Inoue N, Ito S, Itou T, Yasumura M, Yoshinaka Y, Hagita S. et al. Mechanism of lipid-lowering action of the dipeptidyl peptidase-4 inhibitor, anagliptin, in low-density lipoprotein receptor-deficient mice. J Diabetes Investig. 2017;8(2):155–160. doi: 10.1111/jdi.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurozumi A, Okada Y, Arao T, Kobayashi T, Masuda D, Yamashita S, Tanaka Y. Comparison of effects of anagliptin and alogliptin on serum lipid profile in type 2 diabetes mellitus patients. J Diabetes Investig. 2018;9(2):360–365. doi: 10.1111/jdi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arca M, Vega GL, Grundy SM. Hypercholesterolemia in postmenopausal women. Metabolic defects and response to low-dose lovastatin. JAMA. 1994;271(6):453–459. doi: 10.1001/jama.1994.03510300059039. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Miao Z, Liu R, Yang M, Liu H, Yang G. Liraglutide prevents hypoadiponectinemia-induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med. 2011;17(11-12):1168–1178. doi: 10.2119/molmed.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):366–373. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32(6):1513–1519. doi: 10.1161/ATVBAHA.112.246207. [DOI] [PubMed] [Google Scholar]
- 21.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316(7134):823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ. et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 24.Japan Atherosclerosis Society. [Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases] J Atheroscler Thromb. 2007:5–57. [PubMed] [Google Scholar]