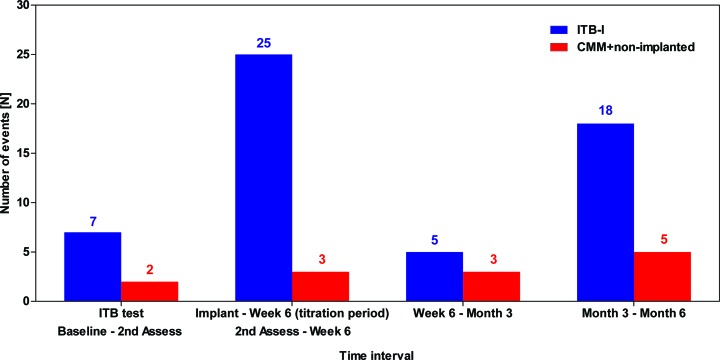
Figure 4.
Frequency of treatment-related AEs (drug, device, procedure) over time during the study (modified ITT population). Second assessment: day 21±2 (CMM arm); week 6: day 44–67 (ITB arm) and corresponds to day 67 for CMM. AE, adverse event; CMM, conventional medical management; ITB, intrathecal baclofen; ITB-I, ITB-implanted; ITT, intention-to-treat.