Abstract
We present the first identification in interstellar space of the thioformyl radical (HCS) and its metastable isomer HSC. These species were detected toward the molecular cloud L483 thanks to observations carried out with the IRAM 30m telescope in the λ 3 mm band. We derive beam-averaged column densities of 7 × 1012 cm−2 for HCS and 1.8 × 1011 cm−2 for HSC, which translate to fractional abundances relative to H2 of 2 × 10−10 and 6 × 10−12, respectively. Although the amount of sulfur locked by these radicals is low, their detection allows to put interesting constraints on the chemistry of sulfur in dark clouds. Interestingly, the H2CS/HCS abundance ratio is found to be quite low, ~ 1, in contrast with the oxygen analogue case, in which the H2CO/HCO abundance ratio is around 10 in dark clouds. Moreover, the radical HCS is found to be more abundant than its oxygen analogue, HCO. The metastable species HOC, the oxygen analogue of HSC, has not been yet observed in space. These observational constraints are confronted with the outcome of a recent model of the chemistry of sulfur in dark clouds. The model underestimates the fractional abundance of HCS by at least one order of magnitude, overestimates the H2CS/HCS abundance ratio, and does not provide an abundance prediction for the metastable isomer HSC. These observations should prompt a revision of the chemistry of sulfur in interstellar clouds.
Keywords: astrochemistry, line: identification, ISM: clouds, ISM: molecules, radio lines: ISM
1. Introduction
The chemistry of sulfur in interstellar clouds has attracted considerable attention from astronomers in the past decades. For example, which is the main reservoir of sulfur in cold dark clouds continues to be an important open question. A variety of simple sulfur-bearing species is observed in the gas phase of these environments. These comprise the hydride H2S, the carbon-bearing molecules CS, H2CS, C2S, and C3S, the oxides SO and SO2, OCS, the nitrogen-bearing species NS, HNCS, and HSCN, and the ion HCS+ (Adande et al. 2010; Agúndez & Wakelam 2013). However, these species only account for less than 0.1 % of the cosmic abundance of sulfur. Moreover, chemical models of cold dark clouds usually need to assume that sulfur is depleted in the gas phase by at least two orders of magnitude to reproduce the abundances derived from observations (Agúndez & Wakelam 2013). However, in photodissociation regions such as the Horsehead Nebula, it is found that sulfur is not severely depleted, only by a factor of 3-4 (Goicoechea et al. 2006). The missing sulfur in cold dark clouds is usually thought to be deposited onto dust grains, although it is not clear in which form. Some sulfur could be trapped in the core of grains as refractory compounds such as FeS or MgS, although it is not known how much sulfur could be in this form. Unfortunately, the depletion factor of sulfur in diffuse interstellar clouds is uncertain because the S+ lines are often saturated (Jenkins 2009). Ices could also be an important reservoir of sulfur in cold dark clouds. Solid OCS and SO2 are thought to be responsible of absorption features in the infrared spectra of young stellar objects, with inferred abundances of 0.2-0.5 % and 0.6-6 %, respectively, of the available sulfur (Palumbo et al. 1997; Boogert et al. 1997). It has been proposed that other ices such as polysulfanes could be a major reservoir of sulfur (Jiménez-Escobar & Muñoz Caro 2011; Druard & Wakelam 2012). It is also possible that gas-phase molecules not yet identified could account for a significant fraction of this element.
The missing sulfur problem has attracted renewed attention in recent years. New potentially important sulfur reservoirs have been explored. For example, the S2-containing molecules H2S2, HS2, and S2 have been unsuccessfully searched for toward the low-mass protostar IRAS 16293-2422 by Martín-Doménech et al. (2016), while the detection of gas-phase H2S in the shocked region L1157-B1 by Holdship et al. (2016) led these authors to conclude that a significant fraction of sulfur must be in the form of H2S ice. The chemistry of sulfur in dark clouds has been recently revisited observationally by Fuente et al. (2016) and theoretically by Vidal et al. (2017). These latter authors point to gasphase atomic sulfur or SH and H2S ices as main sulfur reservoirs. It is also worth noting that two new sulfur-bearing molecules have been recently detected in space: HS2 has been identified in the Horsehead Nebula (Fuente et al. 2017) and the ion NS+ has been ubiquitously found in dense molecular clouds (Cernicharo et al. 2018).
In this Letter we present the first detection in interstellar space of two new sulfur-bearing molecules: the radical HCS and its metastable isomer HSC. These species have been identified in the dense cloud L483, a source that hosts a rich chemistry and where we have recently discovered new molecules like the ketenyl radical (HCCO), protonated cyanogen (NCCNH+), and the ion NS+ (Agúndez et al. 2015a,b; Cernicharo et al. 2018).
2. Observations
The observations were carried out with the IRAM 30m telescope in the frame of a λ 3 mm line survey of L483. We adopted the coordinates of the dense core, δ2000.0 = –04°39′38″, which correspond to the position of the infrared source IRAS 18148–0440 (Fuller & Myers 1993). This position coincides also with the maximum of intensity of CH3OH emission (Tafalla et al. 2000). We used the EMIR receiver E090 in single sideband mode, with image rejections > 10 dB, and employed the frequency-switching technique with a frequency throw of 7.2 MHz. We used the FTS backend in its narrow mode, providing a bandwidth of 1.8 GHz and a spectral resolution of 50 kHz, which translates to velocity resolutions of 0.19-0.13 km s−1 in the 80-116 GHz frequency range. The intensity scale is calibrated using two absorbers at different temperatures and the atmospheric transmission model ATM (Cernicharo 1985; Pardo et al. 2001). We express intensities in terms of the antenna temperature corrected for atmospheric absorption and for antenna ohmic and spillover losses. The uncertainty in is estimated to be around 10 %.
The observations around 81 GHz, where the lines of HCS and HSC presented here lie, were carried out in two observing sessions, during November 2016 and December 2017. Weather conditions were fairly good during these two observing runs, with amounts of precipitable water vapor in the ranges 2.1-5.8 mm and 1.5-2.8 mm, respectively, leading to system temperatures of 85-112 K and 75-92 K, respectively, for elevation angles above 30°. The telescope focus was checked on planets at the beginning of each observing session, which typically lasted ~ 5 h. The telescope pointing was regularly checked every one hour and half by observing the nearby radio source 1741–038. Pointing errors were typically 2-3″. The beam size of the IRAM 30m telescope at 81 GHz is 30″. The on source integration times were 5.05 h in November 2016 and 4.12 h in December 2017. After including data of the two observing sessions and averaging spectra of horizontal and vertical polarizations, the resulting spectrum is very sensitive, with a rms noise level of ~1.5 mK per 50 kHz channel in the spectral region around 81 GHz. The lines of H2CS at 101.5 GHz, 103.0 GHz, and 104.6 GHz were observed in May 2017 in the frame of the same λ 3 mm line survey of L483. Weather conditions were also good, with similar numbers to those given above. More details on these observations will given elsewhere.
3. Results
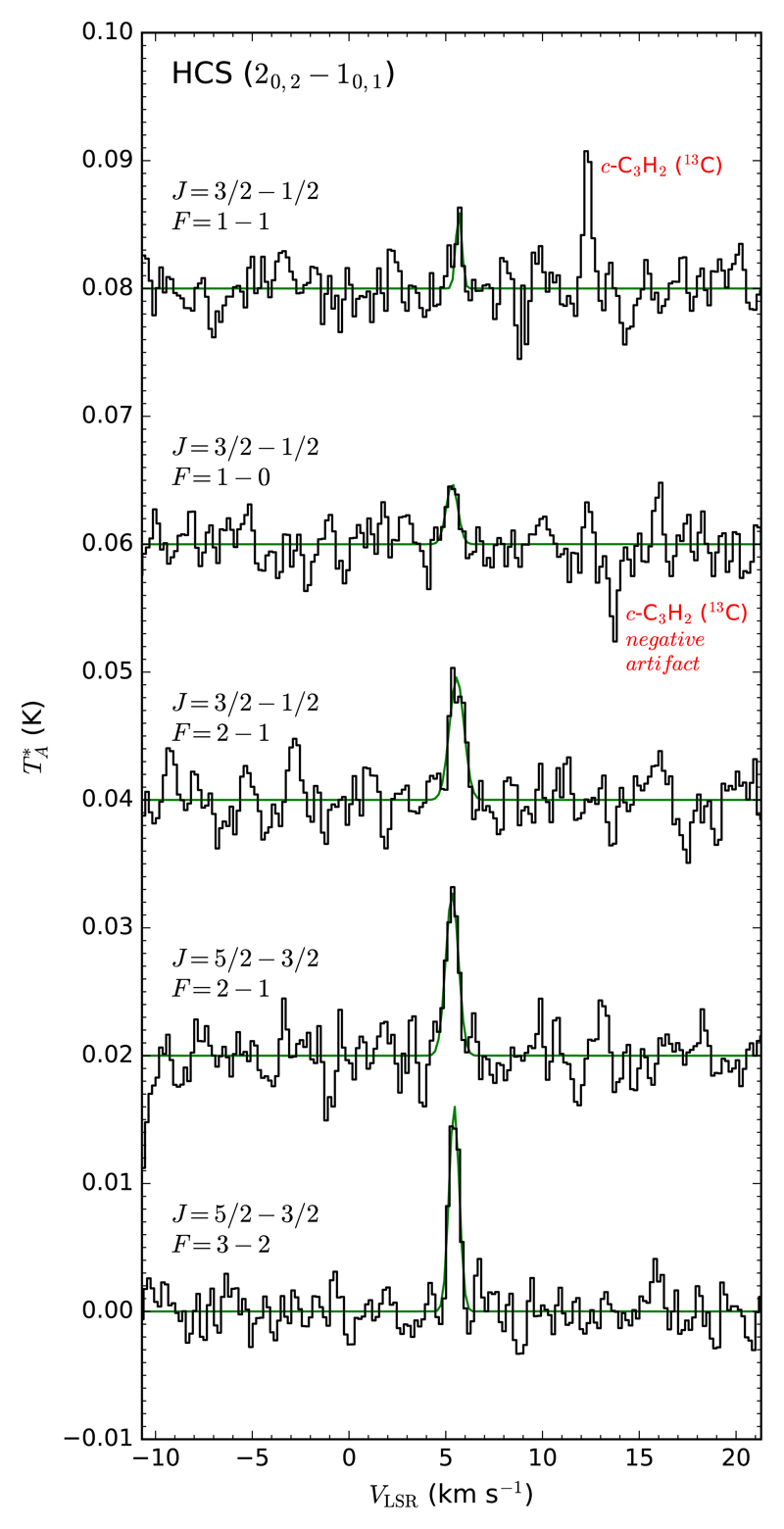
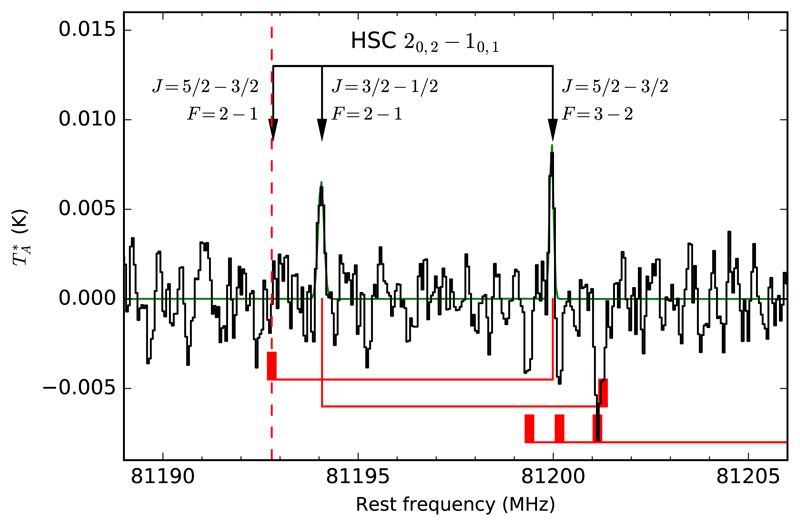
The spectrum of L483 around 80.5 GHz shows a series of emission lines which can be unambiguously assigned to the strongest components of the 20,2 – 10,1 rotational transition of the radical HCS. In the spectral region around 81.2 GHz there are two emission lines whose frequencies precisely coincide with two of the strongest components of the 20,2 – 10,1 rotational transition of the metastable isomer HSC (see Table 1 and Figs. 1 and 2). These species have not been previously observed in space.
Table 1.
Observed line parameters of HCS and HSC in L483.
| Species | Transition | Frequency (MHz) |
Eu (K) |
Aul (s−1) |
gu |
VLSR (km s−1) |
Δv (km s−1) |
(mK km s−1) |
|---|---|---|---|---|---|---|---|---|
| HCS | 20,2 – 10,1 J = 5/2 – 3/2 F = 3 – 2 | 80553.516 | 5.8 | 4.50 × 10−7 | 7 | +5.43(3) | 0.61(5) | 10.5(9) |
| 20,2 – 10,1 J = 5/2 – 3/2 F = 2 – 1 | 80565.596 | 5.8 | 4.34 × 10−7 | 5 | +5.33(5) | 0.77(12) | 10.4(12) | |
| 20,2 – 10,1 J = 3/2 – 1/2 F = 2 – 1 | 80596.409 | 5.8 | 3.41 × 10−7 | 5 | +5.56(6) | 0.88(12) | 9.0(11) | |
| 20,2 – 10,1 J = 3/2 – 1/2 F = 1 – 0 | 80611.994 | 5.8 | 2.51 × 10−7 | 3 | +5.32(10) | 0.70(20) | 3.5(11) | |
| 20,2 – 10,1 J = 3/2 – 1/2 F = 1 – 1 | 80618.820 | 5.8 | 1.79 × 10−7 | 3 | +5.67(7) | 0.36(23) | 2.4(10) | |
| HSC | 20,2 – 10,1 J = 5/2 – 3/2 F = 2 – 1 | 81192.825 | 5.8 | 1.55 × 10−5 | 5 | – | – | – a |
| 20,2 – 10,1 J = 3/2 – 1/2 F = 2 – 1 | 81194.075 | 5.9 | 1.16 × 10−5 | 5 | +5.39(8) | 0.65(15) | 4.5(11) | |
| 20,2 – 10,1 J = 5/2 – 3/2 F = 3 – 2 | 81199.988 | 5.9 | 1.55 × 10−5 | 7 | +5.42(5)b | 0.43(9)b | 4.0(8)b | |
Numbers in parentheses are 1σ uncertainties in units of the last digits. The antenna temperature scale can be converted to main beam brightness temperature by dividing by (Beff/Feff), which is 0.82/0.95 for the IRAM 30m telescope at 81 GHz.
Line is not visible because it overlaps with a frequency switching negative artifact of the HSC 20,2-10,1 J = 5/2 – 3/2 F = 3 – 2 line at 81199.988 MHz (see Fig. 2).
Intensity is reduced because line overlaps with a frequency switching negative artifact of the HSC 20,2-10,1 J = 5/2 – 3/2 F = 2 – 1 line at 81192.825 MHz, line width is likely reduced as well because the line partially overlaps with a negative artifact of a line of CH2CN lying 7.36 MHz higher in frequency (see Fig. 2), and the derived VLSR is probably affected as well.
Fig. 1.
Lines of HCS observed in L483 are shown from bottom to top in order of increasing frequency. Note that the observed line intensity decreases as the frequency increases.
Fig. 2.
Spectra of L483 around 81.2 GHz showing the lines assigned to HSC. The rest frequency scale corresponds to a LSR systemic velocity of +5.3 km s−1. The bottom vertical red marks indicate the position of frequency switching negative artifacts. Note that the J = 5/2 – 3/2 F = 2 – 1 line is not visible because it is artificially cancelled with a frequency switching negative artifact, while the J = 5/2 – 3/2 F = 3 – 2 is also affected by negative artifacts (see text).
The radicals HCS and HSC have a bent structure with a 2A′ ground electronic state. The HSC isomer is ~ 40 kcal mol−1 less stable than HCS (Puzzarini 2005). Their rotational levels NKa,Kc split in a fine (electronic spin-rotation interaction) and hyperfine (H nuclear spin) structure described by the quantum numbers J and F, respectively. The rotational spectra of the two radicals have been characterized in the laboratory (Habara et al. 2002; Habara & Yamamoto 2000), although in the case of the more stable isomer HCS only transitions with Ka = 0 were observed. Line frequencies were obtained from the Cologne Database for Molecular Spectroscopy1 (Müller et al. 2005). The total dipole moments of HCS and HSC have been calculated as 0.96 D and 2.63 D, respectively, with components along the a axis of 0.430 D and 2.493 D, respectively (Puzzarini 2005). We use these latter values for the computation of the column densities because the observed lines correspond to a-type transitions.
The identification of HCS in L483 relies on the detection of a quintet of lines corresponding to the five strongest fine and hyperfine components of the 20,2 – 10,1 rotational transition. The line parameters are given in Table 1 and the lines are shown in Fig. 1. These five transitions have line strengths that decrease with increasing frequency (see Einstein coefficients in Table 1), which is consistent with the observed pattern of line intensities. The brightest line, lying at 80553.516 MHz, has a peak intensity of ~ 15 mK and is well detected above the noise level, with a signal to noise ratio (SNR) ≳ 10σ, while the faintest one, lying at 80618.820 MHz, is observed with a peak intensity of ~ 5 mK and is detected at a lower confidence level, ~ 3σ (see Fig. 1). In the case of the metastable isomer HSC, the 20,2 – 10,1 rotational transition has also multiple components arising from the fine and hyperfine structure. The three strongest ones are those listed in Table 1. Two of these are clearly detected in the spectrum of L483 at confidence levels of 4-5σ, although the one lying at 81192.825 MHz is not visible (see Fig. 2). The most likely reason is that the frequency separation between the components at 81192.825 MHz and 81199.988 MHz is similar to the frequency throw of 7.2 MHz adopted in the frequency switching observations. Therefore, it accidentally happens that each line overlaps with a frequency switching negative artifact of the other line. As a result, in our observed spectrum, the weaker line at 81192.825 MHz is artificially cancelled and the intensity of the stronger line at 81199.988 MHz is also reduced.
Assuming a rotational temperature of 10 K, close to the gas kinetic temperature (Fuller & Myers 1993; Anglada et al. 1997), we derive beam-averaged column densities of 7 × 1012 cm−2 and 1.8 × 1011 cm−2 for HCS and HSC, respectively, in L483. These values translate to fractional abundances relative to H2 of 2 × 10−10 for HCS and 6 × 10−12 for HSC, adopting the value of N(H2) of 3 × 1022 cm−2 derived by Tafalla et al. (2000) from observations of C17O. Note that the column density of HCS only includes Ka = 0 levels. Using the rotational constants A, B, and C from Habara et al. (2002), we estimate that the contribution of Ka ≠ 0 levels to the partition function at 10 K is just 2%. Therefore, the HCS/HSC column density ratio in L483 is ~40. The related molecule H2CS was also observed in the course of the λ 3 mm line survey of L483. For H2CS we estimate excitation temperatures in the range 5-10 K, adopting a volume density of H2 of 3.4 × 104 cm−3 (Jørgensen et al. 2002) and taking into account that H2CS should be excited through collisions more efficiently than H2CO due to the larger geometrical cross section. From the observed intensities and assuming rotational temperatures in the range 5-10 K, we derive a beam-averaged column density of (1.0 – 2.1) × 1013 cm−2 for H2CS.
Therefore, the radical HCS has an abundance similar to that of thioformaldehyde, with a H2CS/HCS column density ratio of 1.4-3, i.e., of the order of unity. The situation here is markedly different from that of the oxygen-bearing analogues. In L483, the H2CO/HCO column density ratio is 19 (Tafalla et al. 2000; Agúndez et al. 2015a), in line with the values found in other cold dark clouds, which are in the range 7-22 (see Ocaña et al. 2017 and references therein). Therefore, while the radical HCO is around ten times less abundant than H2CO in cold dark clouds, in L483 the HCS radical is as abundant as H2CS. Moreover, HCS is even more abundant than HCO in L483. The presence of the metastable isomer HSC in cold dark clouds makes also a difference between oxygen and sulfur chemistries. The oxygen analogue HOC has not been observed in space, essentially because there is a complete lack of experimental information for this species. Both metastable isomers HOC and HSC lie ~40 kcal mol−1 above their respective stable isomers, HCO and HCS. However, HOC lies also above the fragments H and CO, whereas HSC is more stable than H and CS (Marenich & Boggs 2003; Puzzarini 2005; Pérez-Juste & Carballeira 2007). Still, the metastable isomer HOC should be stable enough to be characterized because the decay to H and CO is predicted to have a barrier (Marenich & Boggs 2003). The search for HOC in space must in any case await its detection in the laboratory.
4. Discussion
The fractional abundances relative to H2 derived for HCS and HSC, a few 10−10 and several 10−12, respectively, indicate that these species do not lock an important fraction of sulfur. However, the finding that HCS has an abundance similar to H2CS and the detection of the metastable isomer HSC put interesting constraints on the chemistry of sulfur in cold dark clouds.
The chemistry of sulfur in dark clouds has been recently revisited by Vidal et al. (2017) in a study in which the sulfur chemical network was thoroughly revised. Interestingly, this study discusses the potential detectability of HCS based on the non-negligible abundance predicted for this radical. The calculated abundance between 105 and 106 yr is however in the range 10−12-10−11 relative to H2, which is below the observed value by at least one order of magnitude. Moreover, the observational finding of a H2CS/HCS abundance ratio close to 1 is not well accounted for by their model, which predicts that H2CS is one to two orders of magnitude more abundant than HCS. No prediction is made for the metastable isomer HSC as this species is not included in their model.
The main reactions leading to HCS in the model of Vidal et al. (2017) are
| (1) |
| (2) |
| (3) |
| (4) |
Reactions (1), (2), and (3) are the sulfur analogues of the main reactions forming the HCO radical in cold dark clouds, in which case chemical models calculate an abundance in good agreement with observed values (Agúndez et al. 2015a; Bacmann & Faure 2016; Ocaña et al. 2017). Observations however indicate that the oxygen and sulfur cases behave differently, as indicated by the different abundance ratios: H2CO/HCO > 1 and H2CS/HCS ~ 1. Reaction (1) is basically considered by similarity with the O + CH2 reaction, which has been suggested to yield HCO. Note however that it is not fully clear whether this reaction can proceed (Ocaña et al. 2017), and thus it would be of great interest to study theoretically the reactions of O and S atoms with CH2 to evaluate their role in the formation of HCO and HCS, respectively, and of their metastable isomers HOC and HSC. Reaction (2) is a feasible route to HCS, although since the most stable structure of the ion H2CS+ has the two hydrogen atoms bonded to carbon (Curtis et al. 1992), it would require an important rearrangement to yield HSC. Reaction (3) is a likely pathway to HCS. Moreover, the similar abundances derived for H2CS and HCS are suggestive of a common origin and thus the dissociative recombination of H3CS+ could be a common source of both species. Reaction (4) has been studied experimentally and theoretically and offers an interesting route to both HCS and HSC. The reaction is rapid at room temperature, with a measured rate constant of 2.5 × 10−10 cm3 s−1 (Deeyamulla & Husain 2006). Theoretical calculations indicate that both HCS and HSC can form without energy barrier (Ochsenfeld et al. 1999; Galland et al. 2001), although the formation of HCS is more exothermic (40-44 kcal mol−1) that that of HSC (just 2-4 kcal mol−1), which could favor HCS over HSC. In fact, this would be in agreement with crossed-beam experiments (Kaiser et al. 1999; Ochsenfeld et al. 1999). Note that although reactions (1-4) are plausible routes to HCS, their implementation in the model of Vidal et al. (2017) is not enough to rise the abundance of this radical up to the level observed in L483. It is clear that the chemistry of sulfur still needs to be revised in the light of the new observational constraints provided by the detection of HCS and HSC.
5. Conclusions
We have presented the first detection in space of the thioformyl radical (HCS) and its metastable isomer HSC. These species have been observed in the molecular cloud L483, with fractional abundances relative to H2 of a few 10−10 for HCS and various 10−12 for HSC. The fraction of sulfur locked by these radicals is low. However, their detection puts interesting constraints on the chemistry of sulfur in dark clouds. We find significant differences with respect to the oxygen analogue species. First, the observed H2CS/HCS abundance ratio is close to unity, while the H2CO/HCO abundance ratio in dark clouds is found to be around 10. Second, the metastable isomer HSC is found with a low abundance, while its oxygen analogue HOC has not been yet observed in space. The latest chemical models of dark clouds cannot account for the relative abundance of HCS, the H2CS/HCS ratio, and the presence of HSC, suggesting that a revision of the chemistry of sulfur is required.
Acknowledgements
We thank the referee for a careful reading of the manuscript and the IRAM 30m staff for their help during the observations. We acknowledge funding support from the European Research Council (ERC Grant 610256: NANOCOSMOS) and from Spanish MINECO through grant AYA2016-75066-C2-1-P. M.A. also thanks funding support from the Ramón y Cajal programme of Spanish MINECO (RyC-2014-16277).
Footnotes
References
- Adande GR, Halfen DT, Ziurys LM, et al. ApJ. 2010;725:561. [Google Scholar]
- Agúndez M, Wakelam V. Chem Rev. 2013;113:8710. doi: 10.1021/cr4001176. [DOI] [PubMed] [Google Scholar]
- Agúndez M, Cernicharo J, Guélin M. A&A. 2015a;577:L5. doi: 10.1051/0004-6361/201526317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agúndez M, de Vicente P, et al. A&A. 2015b;579:L10. [Google Scholar]
- Anglada G, Sepúlveda I, Gómez JF. A&AS. 1997;121:255. [Google Scholar]
- Bacmann A, Faure A. A&A. 2016;587:A130. [Google Scholar]
- Boogert ACA, Schutte WA, Helmich FP, et al. A&A. 1997;317:929. [Google Scholar]
- Cernicharo J. IRAM Internal Report 52. 1985 [Google Scholar]
- Cernicharo J, Lefloch B, Agúndez M, et al. ApJ. 2018;853:L22. doi: 10.3847/2041-8213/aaa83a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss LA, Nobes RH, Pople JA, Radom L. J Chem Phys. 1992;97:6766. [Google Scholar]
- Deeyamulla MP, Husain D. J Photochem Photobiol A: Chem. 2006;184:347. [Google Scholar]
- Druard C, Wakelam V. MNRAS. 2012;426:354. [Google Scholar]
- Fuente A, Cernicharo J, Roueff E, et al. A&A. 2016;593:A94. doi: 10.1051/0004-6361/201628285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente A, Goicoechea JR, Pety J, et al. ApJ. 2016;851:L49. [Google Scholar]
- Fuller GA, Myers PC. ApJ. 1993;418:273. [Google Scholar]
- Galland N, Caralp F, Rayez M-T, et al. J Phys Chem A. 2001;105:9893. [Google Scholar]
- Goicoechea JR, Pety J, Gerin M, et al. A&A. 2006;456:565. [Google Scholar]
- Habara H, Yamamoto S. J Chem Phys. 2000;112:10905. [Google Scholar]
- Habara H, Yamamoto S, Amano T. J Chem Phys. 2002;116:9232. [Google Scholar]
- Holdship J, Viti S, Jiménez-Serra I, et al. MNRAS. 2016;463:802. [Google Scholar]
- Jenkins EB. ApJ. 2009;700:1299. [Google Scholar]
- Jiménez-Escobar A, Muñoz Caro GM. A&A. 2011;536:A91. [Google Scholar]
- Jørgensen JK, Schöier FL, van Dishoeck EF. A&AS. 2002;389:908. [Google Scholar]
- Kaiser RI, Ochsenfeld C, Head-Gordon M, Lee YT. J Chem Phys. 1999;110:2391. [Google Scholar]
- Marenich AV, Boggs JE. J Phys Chem A. 107:2343. doi: 10.1021/jp070495f. [DOI] [PubMed] [Google Scholar]
- Martín-Doménech R, Jiménez-Serra I, Muñoz Caro GM, et al. A&A. 2016;585:A112. [Google Scholar]
- Müller HSP, Schlöder F, Stutzki J, Winnewisser G. J Mol Struct. 2005;742:215. [Google Scholar]
- Ocaña AJ, Jiménez E, Ballesteros B, et al. ApJ. 2017;850:28. doi: 10.3847/1538-4357/aa93d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenfeld C, Kaiser RI, Lee YT, Head-Gordon M. J Chem Phys. 1999;110:9982. [Google Scholar]
- Palumbo ME, Geballe TR, Tielens AGGM. ApJ. 1997;479:839. [Google Scholar]
- Pardo JR, Cernicharo J, Serabyn E. IEEE Trans Antennas Propag. 2001;49:1683. [Google Scholar]
- Pérez-Juste I, Carballeira L. J Chem Phys. 2007;127 doi: 10.1063/1.2777138. 164303. [DOI] [PubMed] [Google Scholar]
- Puzzarini C. J Chem Phys. 2005;123 doi: 10.1063/1.1953367. 024313. [DOI] [PubMed] [Google Scholar]
- Tafalla M, Myers PC, Mardones D, Bachiller R. A&A. 2000;359:967. [Google Scholar]
- Vidal THG, Loison J-C, Yassin Jaziri A, et al. MNRAS. 2017;469:435. [Google Scholar]