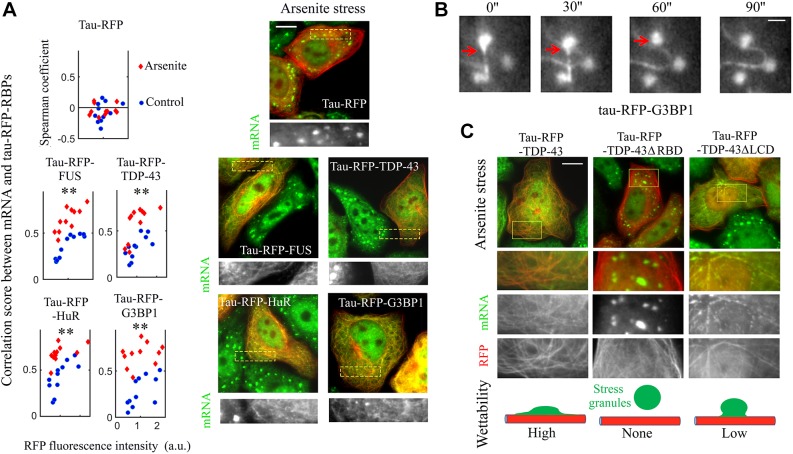
Fig. 2.
Tau–RFP–RBPs colocalize with mRNA on microtubules and lead to the wetting of stress granules on microtubules. (A) Right panel, spatial distribution of mRNA and tau–RFP–RBP in arsenite-treated cells (300 µM, 60 min). Arsenite leads to the formation of stress granules in control cells. Tau–RFP alone does not interact with stress granules. Fluorescent poly(T) probes were used to detect mRNA (green). Scale bar: 10 µm. Left panel: colocalization score between tau–RFP–RBPs and mRNA in control and arsenite-treated cells plotted against the tau–RFP–RBP expression levels (a.u., arbitrary units). The colocalization score correlates with tau–RFP–RBP expression levels. Arsenite further increases the colocalization score. Three independent experiments were performed, and led to the same observation. Colocalization analysis was performed as described in the Materials and Methods. Each dot represents a measurement from a single cell (ncell=11). **P<0.01 for arsenite-treated versus control cells (two-tailed t-test). (B) Time-lapse images (time in seconds) of tau–RFP–G3BP1 (Movie 2). Cells were treated with arsenite (300 µM) and nocodazole (500 nM) for 60 min. The red arrow indicates the interactions of a G3BP1 compartment on microtubules with stress granules. Scale bar: 2 µm. (C) Tau–RFP was fused to full-length or truncated TDP-43 in arsenite-treated cells. Either removing the LCD or RBD of TDP-43 alters the wetting of stress granules on microtubules, as summarized in the diagram in the lower panel. Scale bar: 10 µm.