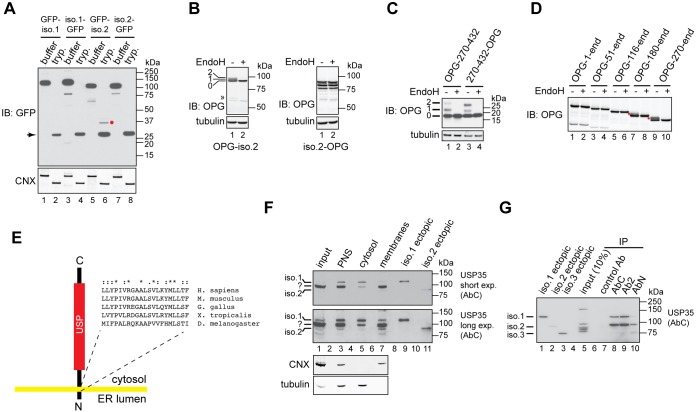
Fig. 3.
USP35 isoform 2 is a novel integral membrane DUB. (A) Expression of the indicated variants of USP35 in U2OS FlpIn cells was induced with tetracycline, cells were semi-permeabilised with digitonin and trypsin (tryp.) or buffer control was added. Samples were incubated for 1 h on ice, resolved by SDS-PAGE and immunoblotted (IB) with anti-GFP antibody. Immunoblotting for calnexin (CNX) was used to validate the assay. The red dot indicates the protease-resistant USP35 fragment; the arrow marks a protein species most likely corresponding to high amount of trypsin used in the assay. (B) U2OS FlpIn cells were transfected with USP35iso2 tagged at the N- or C-terminus with a peptide derived from bovine rhodopsin (OPG) that contains two N-glycosylation sites. Cells were lysed, and samples incubated with EndoH and resolved by SDS-PAGE. Results were visualised by immunoblotting with an anti-rhodopsin antibody. Numbers on the left indicate the number of N-glycans added to USP35iso2; the double arrowhead indicates an EndoH-sensitive truncated product of N-terminally tagged USP35iso2. (C) As in B, but an USP35 truncation spanning residues 270–432 was used. Numbers on the left indicate the number of N-glycans attached to the USP35 fragment. (D) As in B, but the indicated truncations of USP35 bearing an N-terminal OPG tag were used. Note that isoform 2 corresponds to the ‘270-end’ variant. Red dots represent N-glycosylated species. (E) Experimentally validated topology of USP35iso2. Amino acid sequence and conservation of the predicted transmembrane region is shown. ‘*’, fully conserved residues; ‘:’, conserved residues of strong groups; ‘.’, conserved residues of weak groups, as based on ClustalX. The sequences used were as follows: NP_065849.1 (H. sapiens), NP_001170883.1 (M. musculus), XP_015136357.1 (G. gallus), XP_002936594.2 (X. tropicalis) and NP_725167.1 (D. melanogaster). (F) HPAF-II cells were lysed in the absence of detergent, and post-nuclear supernatant (PNS), cytosolic and membrane fractions were isolated. Endogenous USP35 was identified by immunoblotting using the anti-USP35 antibody raised against the extreme C-terminus of the protein. Lysates of HPAF-II cells transfected with untagged USP35 isoforms 1 and 2 were used as markers for the electrophoretic migration of USP35 isoforms. CNX and tubulin were used as markers for the membrane and cytosolic fractions, respectively. Short and long exposures of the membrane are shown. (G) HPAF-II cells were lysed in RIPA buffer, and USP35 immunoprecipitated (IP) by using the indicated antibodies. Samples were resolved by SDS-PAGE and immunoblotted with the anti-USP35 antibody recognising the C-terminal region of the protein. Ectopically expressed untagged USP35 isoforms were used as markers for the electrophoretic mobility.