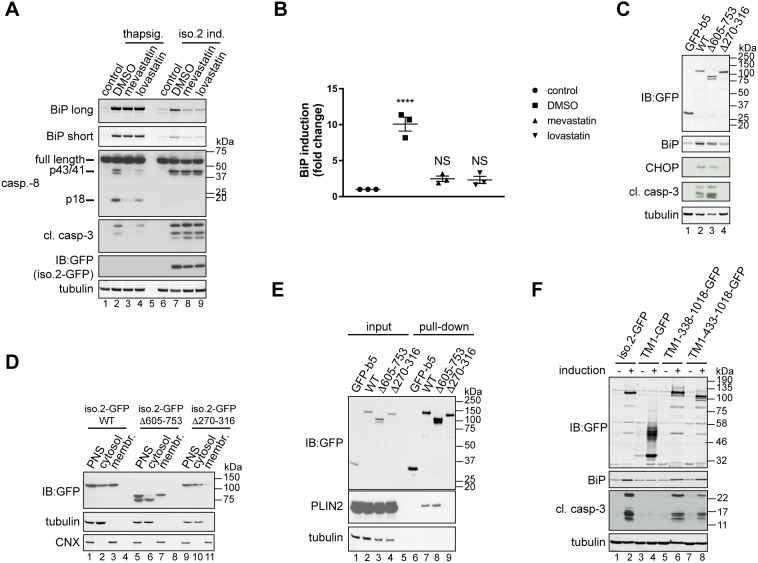
Fig. 5.
Inhibition of cholesterol biosynthesis blocks USP35iso2-induced ER stress. (A) U2OS FlpIn parental cells were pre-treated with 10 µM mevastatin or lovastatin for 2 h and incubated with 100 nM thapsigargin for an additional 18 h (lanes 2–4). In parallel, U2OS FlpIn cells were induced (ind.) to express USP35iso2 for 48 h in the presence of 10 µM statins. Samples were analysed as in Fig. 4B. Control samples represent cells without thapsigargin treatment or USP35iso2 induction. A long and short exposure is shown for the BiP blot. (B) Samples after statin treatment and USP35iso2 induction as shown in A (lanes 6–9) were analysed by quantitative western blotting, and the fold change of BiP induction relative to control plotted. Error bars indicate the s.e.m. with n=3 biological replicates. ****P<0.0001; NS, not significant (one-way ANOVA test). (C) As in Fig. 4B, but U2OS FlpIn cells expressing indicated variants of USP35iso2 in a tetracycline-inducible manner were used. (D) U2OS FlpIn cells expressing indicated variants of USP35iso2 for 24 h were subjected to subcellular fractionation followed by western blotting analysis. WT, wild-type. (E) U2OS FlpIn cells were induced to express indicated GFP-tagged variants of USP35iso2 or GFP-b5 for 24 h. Cells were lysed, and GFP-tagged proteins and their binding partners recovered and samples analysed by western blotting against the indicated proteins. (F) The indicated USP35 fragments (numbering based on isoform 1) were tagged at the C-terminus with GFP and at the N-terminus with the first 61 residues of SPP, which encompasses its first transmembrane region (TM1). USP35iso2 and GFP fused to the same N-terminal fragment of SPP served as controls. Cells were treated and samples processed as described for Fig. 4B.