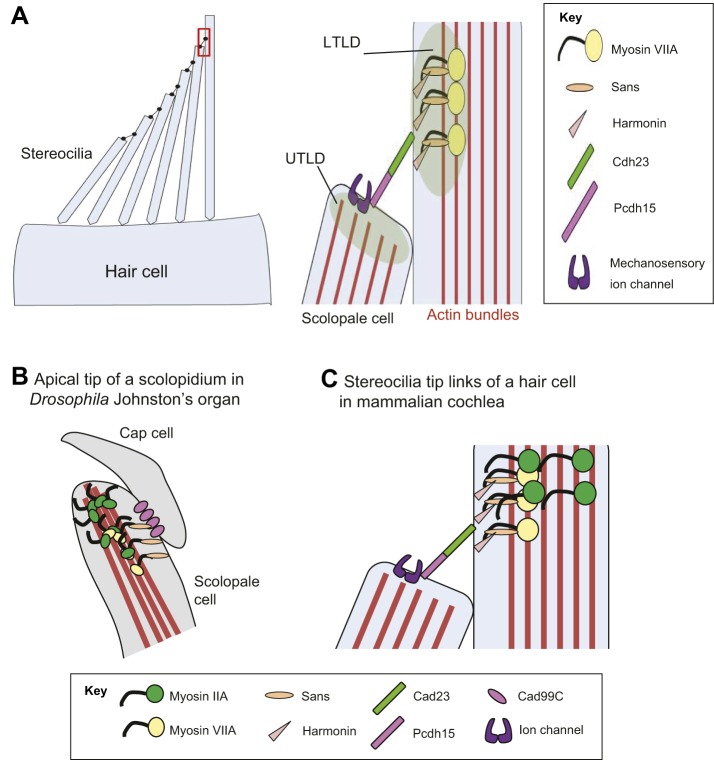
Fig. 3.
USH1 proteins and Myosin II function in the apical region of Drosophila scolopidia and mammalian stereocilia. (A) Left: a schematic of the apical surface of a hair bundle in a single mouse hair cell, showing individual stereocilia. The red box outlines the tip link region, which is expanded to high magnification on the right. Right: USH proteins form a protein complex and link the upper tip link density (UTLD) in the taller stereocilia to the lower tip link density (LTLD) of the neighboring shorter stereocilia in the same hair cell. In the prevailing model of USH protein interactions, the Cdh23-Pchd15 heterodimer is located directly or in close proximity to other protein components of the mechanotransduction (MET) channel complex, including TMIE and TMHS. The adaptor proteins harmonin and Sans link Cdh23 to myosin VIIA and hence to the actin core of the stereocilium. (B) A schematic of a scolopidium. In Drosophila, Myosin VIIA and Myosin II are present in the apical regions of the scolopidia of Johnston's organ and are enriched at the tips of scolopale cells, where they contact the cap cell. Myosin II ubiquitination promotes its interaction with Myosin VIIA, the levels of which are crucial for anchoring the apical junction complexes of the scolopidia. The motor activity of these myosins might also be required to transport the myosin complex to the tips of the scolopale cell. Both Myosin VIIA and Myosin II likely bind to actin bundles in the scolopale cells and regulate the apical attachment of scolopidia. Two Drosophila homologs of Usher syndrome type I proteins, Cad99C (Pcdh15) and Sans, interact with Myosin VIIA in a protein complex. It is not clear whether Cad99C mediates attachment to neuronal cilia or cap cells as a homodimer or as a heterodimer with another adhesion molecule. (C) In mammalian hair cells, a USH1 protein complex that includes myosin VIIA, Sans, harmonin and Cdh23 is located close to the stereocilia tips. By analogy with Drosophila, we propose that myosin IIA interacts with myosin VIIA, and that this interaction is promoted by myosin II ubiquitination. The motor activity of either myosin might be required for the transport of the myosin VIIA-myosin II-USH1 protein complex to the stereocilia tips.