Figure 1.
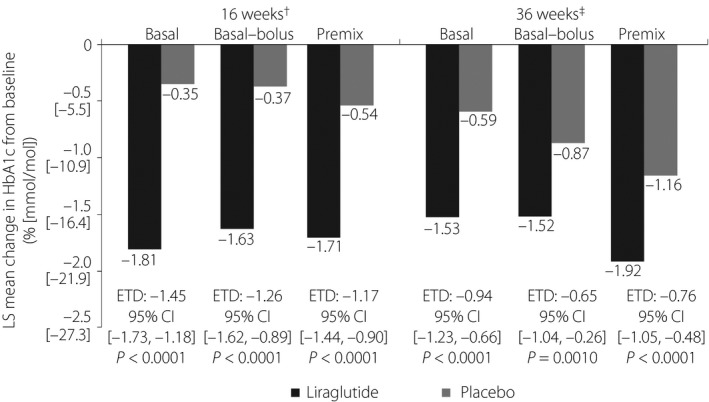
Change in glycated hemoglobin (HbA1c; % [mmol/mol]) from baseline in participants receiving liraglutide or a placebo in addition to basal insulin (n = 100), basal–bolus insulin (n = 55) or premix insulin therapy (n = 102), after 16 and 36 weeks of treatment. The analysis of efficacy end‐points was based on the full analysis set, defined as all randomized participants who received at least one dose of trial product, with each participant contributing as randomized. †Test for interaction P = 0.3353 between treatment and pre‐trial insulin at 16 weeks. ‡Test for interaction P = 0.4511 between treatment and pre‐trial insulin at 36 weeks. CI, confidence interval; ETD, estimated treatment difference; LS, least squares.
