Figure 2.
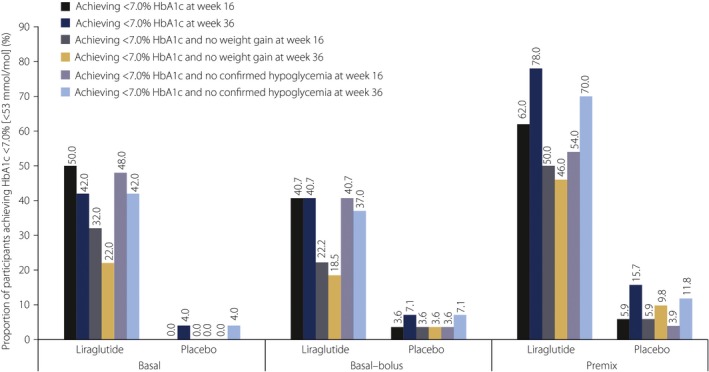
Proportion of participants who achieved glycated hemoglobin (HbA1c) <7.0% (<53 mmol/mol), HbA1c <7.0% with no weight gain and HbA1c <7.0% with no confirmed hypoglycemia (after 16 and 36 weeks of treatment) with addition of liraglutide or placebo to basal insulin (n = 100), basal–bolus insulin (n = 55) or premix insulin therapy (n = 102). The analysis of efficacy end‐points was based on the full analysis set defined as all randomized participants who received at least one dose of the trial product, with each participant contributing as randomized.
