Abstract
Acute myeloid leukemia (AML) is a blood disorder characterized by uncontrolled proliferation of myeloid progenitors and decrease in the apoptosis rate. The vascular endothelial growth factor (VEGF) promotes blood vessel regeneration which might play important roles in development and progression of neoplasia. Our previous studies focused on cytotoxicity and anticancer effects of arsenic trioxide (ATO) and thalidomide (THAL) as an anti-VEGF compound in the AML cell model. ATO also affects regulatory genes involved in cell proliferation and apoptosis. The aim of present study was to examine the effects of ATO and THAL alone and in combination on U937 and KG-1 cells, with attention to mRNA expression for VEGF isoforms. Growth inhibitory effects was assessed by MTT assay and apoptosis induction was determined by Annexin/PI staining. mRNA expression levels were evaluated by real-time PCR. Our data indicated that ATO (1.618μM and 1μM in KG-1 and U937 cell lines respectively), THAL (80μM and 60μM) and their combination inhibited proliferation and induced apoptosis in our cell lines. mRNA expression of VEGF (A, B) decreased while C and D isoforms did not show any significant changes. Taken together, according to the obtained results, the VEGF autocrine loop could be a target as a therapeutic strategy for cases of AML.
Keywords: Arsenic trioxide, Thalidomide, Vascular Endothelial Growth Factor (VEGF), acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is the heterogeneous malignant which is characterized by the uncontrolled proliferation of hematopoietic stem cells and myeloid (Döhner et al., 2015; Mohammadi et al., 2016). Although with conventional AML regiment many patients initially achieve remission, but eventually relapse occur due to chemotherapy evaded leukemic stem cells (Mohammadi et al., 2017a; Mohammadi et al., 2017b). Angiogenesis is a regulated process, which creates new blood vessels from a pre-existing vascular network (Kerbel, 2008), and plays an important role in the progression of hematolymphoid malignancies. Vascular Endothelial Growth Factor (VEGF) is a 46 KD heparin-binding homodimer protein including six different isoforms namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and VEGF-F (Tischer et al., 1991; Lei et al., 1998; Mirzaei et al., 2017). In fact, it plays a necessary role in the developmental, physiological and pathological angiogenesis (Ferrara and Davis-Smyth, 1997). VEGF-A is the prototype which is commonly known as the VEGF. All of these ligands have binding with three transmembrane receptor tyrosine kinases including VEGFR-1, VEGFR-2 and VEGFR-3, and they develop the endothelium regeneration and increase the vascular permeability (Haghi et al., 2017). The secreted VEGF by leukemic cells interacts with relevant receptors on the endothelial cell surface and stimulates endothelial cells to produce growth factors, which result in an increase in their proliferative activities and drug resistance. The anti-angiogenesis therapy is based on inhibiting the physiological function of VEGF recognized as a new therapeutic strategy (Rafii et al., 2002; Mirzaei et al., 2017). Arsenic trioxide (ATO) has been used to treat different types of malignancies (Rodriguez-Ariza et al., 2011). ATO has numerous biological effects such as infraction of mitochondrial respiration, depletion of cellular thiols, and effects on the apoptosis and anti-proliferative activities (Miller et al., 2002). ATO impels the expression of Bax which causes down-regulated expression of Bcl-2 family members and inhibits the NF-κB activation (Miller et al., 2002). In addition, ATO prevents the angiogenesis by inhibiting the cell growth (Lew et al., 1999). ATO causes down-regulation of VEGF expression and increases the apoptosis (Roboz et al., 2000) (Figure 1). THAL has anti-angiogenesis effects on tumour growth and progression (Woodyatt, 1962; Salemi et al., 2017). This agent inhibits the angiogenesis of basic fibroblast growth factor (β-FGF) in rabbit and VEGF in murine (D’Amato et al., 1994; Kenyon et al., 1997). Due to the anti-angiogenesis property of THAL, it has been used for treatment of various solid tumours, multiple myeloma, and other hematologic malignancies (Figg et al., 1997; Eisen et al., 1998; Long et al., 1998; Marx et al., 1999; Drake et al., 2003). (Figure 2) Hence, the aim of this study was to evaluate the combination effects of ATO and THAL as a new strategy with anti-VEGF properties and induction of apoptosis in leukemic cell lines.
Figure 1.
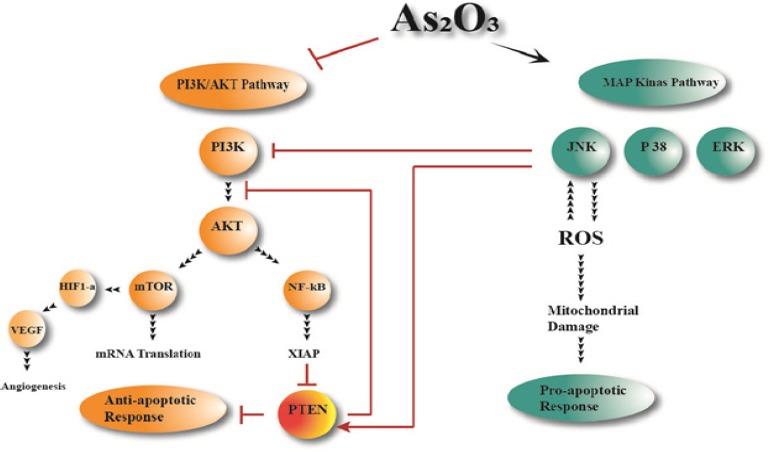
Targeting of Signaling Pathways by ATO in AML Cells. ATO treatment of leukemic cells results in inhibition of the PI3K/Akt pathway; and pharmacologic targeting of this pathway enhances the antileukemic effects of ATO. The potential involvement of other MAPK pathways, such as the p38 MAPK and MEK/ERK pathways, which play important roles in the control of growth and survival of other types of leukemic cells. ATO could suppress angiogenesis factor indirectly by suppress PI3K/AKT pathway.
Figure 2.
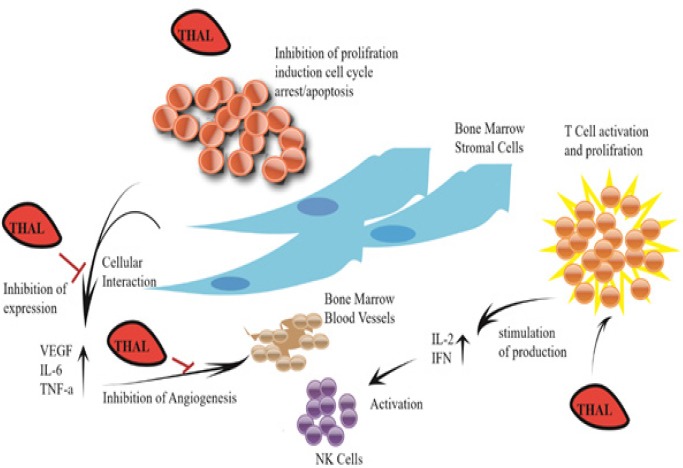
Mechanism of Anti-Angiogenic and Immunomodulatory Actions of Thalidomide (THAL). thalidomide metabolites upon oxidation, inhibits interleukin 6 and TNFα, precluding its nuclear translocation and induction of genes that function in metastasis, angiogenesis, cellular proliferation, inflammation, and protection from apoptosis. THAL metabolites also function in T cell activation as its T cell costimulatory function, enhancing T cell proliferation. The activated T cells release interleukin-2 (IL-2) and interferon-gamma (IFN-γ), which activate the Natural Killer cells (NK cells).
Materials and Methods
Reagents
THAL was purchased from Santa Cruz Company (Santa Cruz, Dallas, Texas); and ATO was obtained from Sina Darou Company (Tehran, Iran). 5-diphenyltetrazolium bromide (MTT) dye, Annexin V-FITC apoptosis detection kit, Dimethyl Sulfoxide (DMSO) and DEPC treated water were purchased from Sigma-Aldrich Company (Sigma-Aldrich, St. Louis, MO). RPMI 1640 medium and fetal bovine serum (FBS) were obtained from Gibco (Gibco, Carlsbad, CA). The cDNA synthesis kit and SYBR Premix Ex Taq™ were bought from Takara Biotechnology Co. (Takara Bio Inc., Otsu, Japan). And TRI Pure isolation Reagent was obtained from Roche Applied Science Company (Roche Applied Science, Germany).
Cell lines and cell culture
KG-1 and U937 were purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). U937 cells were cultured in RPMI 1640 medium which was supplemented with 10% FBS, 100 units/mL of Penicillin and 100 µg/mL of Streptomycin. KG-1 cells were cultured in the RPMI 1640 medium which was supplemented with 20% FBS, 100 units/ml of Penicillin and 100 µg/ml of Streptomycin. Cells were then cultured at 37°C in a humidified atmosphere containing 5% CO2. THAL was dissolved in DMSO, and then dissolved in sterile double-distilled water. ATO was dissolved in distilled water.
Microculture Tetrazolium Test (MTT)
U937 and KG-1 cell line were seeded (at an initial density 5 × 103 cells per well) in 96-well plate. Cells were treated with ATO, Sorafenib and their combinations at 37ºC and 5% CO2 atmosphere for 24, 48 and 72 hours. The proliferation rate of cells was analyzed by MTT assay. Results were expressed as a proliferation rate, with 100% representing control cells treated with 0.1% DMSO alone.
Apoptosis assay
U937 and KG-1 cell lines were seeded at density of 3 × 105 cell/well and incubated for 48 hours in absence and presence of ATO, Sorafenib and their combinations. To assess the percentage of apoptosis induction by treated compounds, fluorescein-conjugated Annexin V (Annexin V-FITC) staining assay was accomplished based on manufacturer protocol. The percentage of apoptosis was determined as a percentage of the Annexin V+/PI- cells through flow cytometry by BD flow cytometer instrument and analyzed with flowjo (Tree Star Inc., version 9.6.3, USA) program.
Cell Cycle Flow cytometry Analysis
Cells were treated with indicated concentrations of ATO and Sorafenib for 48h, then fixed in 70% in ethanol and dye by PI. Cells were assessed by BD flow cytometer instrument analyzed with flowjo program. The apoptotic cell fraction could predict from hypodiploid sub-G0/G1 DNA fraction.
RNA isolation and Real-time PCR
Total RNA was extracted by using the TRI pure (Roche Applied Science, Germany), according to the manufacturer instructions. The RNA pellets were reconstituted in DEPC treated water. The quality and quantity of total RNA was determined spectro-photometrically using Nanodrop ND-1,000 (Nanodrop Technologies, Wilmington, DE) at 260 and 280 nm. Complementary DNAs (cDNAs) were reverse transcribed from 1-2µg of total RNA by use of cDNA synthesis kit (Takara Bio Inc., Otsu, Japan) according to the manufacturer instructions. The cDNA concentration was then normalized in series of PCR by using HPRT and GAPDH primers (Table-3). The normalized cDNAs were subjected to amplification, using Step One Plus™ ABI instrument (Apply Bio systems, USA). The levels of HPRT mRNA expression were used to estimate the relative expression levels. The comparative Ct method was used to compute relative expression values. The primers and their corresponding amplicon lengths were provided in Table 1.
Table 1.
Primer Used for qRT -PCR
| Gene | Forward Primer(5’-3’) | Reverse Primer(5’-3’) | Size(bp) | Ref |
|---|---|---|---|---|
| GAPDH | TGAACGGGAAGCTCACTGG | TCCACCACCCTGTTGCTGTA | 19 | (Kong et al., 2014b) |
| HPRT | GCTATAAATTCTTTGCTGACCTGCTG | AATTACTTTTATGTCCCCTGTTGACTG | 26 | (Gusenbauer et al., 2015) |
| VEGF-A | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA | 21 | (Kong et al., 2014a) |
| VEGF-B | GAGATGTCCCTGGAAGAACACA | GAGTGGGATGGGTGATGTCAG | 22 | (Yang et al., 2009) |
| VEGF-C | GAGGAGCAGTTACGGTCTGTG | TCCTTTCCTTAGCTGACACTTGT | 21 | (Awad et al., 2015) |
| VEGF-D | GTATGGACTCTCGCTCAGCAT | AGGCTCTCTTCATTGCAACAG | 21 | Takeshi Terabayashi et al., 2015) |
Data analysis) Statistical analysis
All in vitro experiments were performed in triplicate, and results have been expressed as the mean±standard deviation (SE). Student’s t test and one-way analysis of variance (one-way ANOVA) were used to determine statistical significances of difference. Statistical significance were defined at *P<0.05, **P<0.01, and ***P<0.001 compared to corresponding control.
Results
Cell proliferation
MTT assay was used to assessed the inhibitory effects of ATO and THAL on cell proliferation after 24, 48 and 72 hours of treatment with different concentrations of ATO (0.4 - 5 µM) and THAL (5-100µM). Results of MTT indicated that ATO inhibited cell proliferation by the lowest IC50 value 1.618 µM and 1 µM for KG-1 and U937 cells respectively. The IC50 value of THAL was 80 µM and 60µM for KG-1 and U937 correspondingly. Our result demonstrated that the combination of these compounds significantly reduced the proliferation of cited leukemic cells. ATO and THAL reduce proliferation of KG-1 and U937 in a dose-dependent manner (Figure 3: A-C and Figure 4: A-C).
Figure 3.
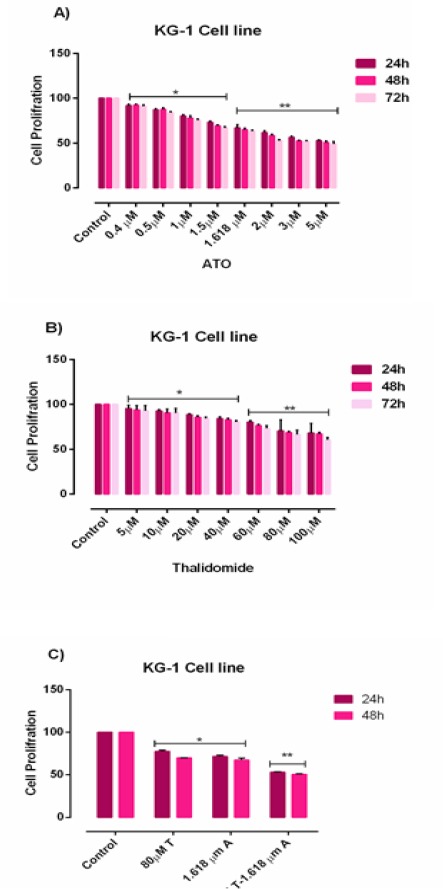
Cell Proliferation in KG-1. Effects of ATO and THAL on cell proliferation of KG-1 cell lines. The anti-proliferative effects of ATO (0-5 μM), THAL (0-100 μM) and their combinations on KG-1 were assessed by MTT assay after 24 h-48h and 72 h treatment. Significant difference between 48h and 72h was not observed. After detection of suitable doses for ATO (1.618μM) and THAL (80μM), we evaluated combination effect of ATO and THAL. Combination effect of ATO and THAL compared to the control or even single compound could significantly decrease cell proliferation in KG-1 cell lines. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Figure 4.
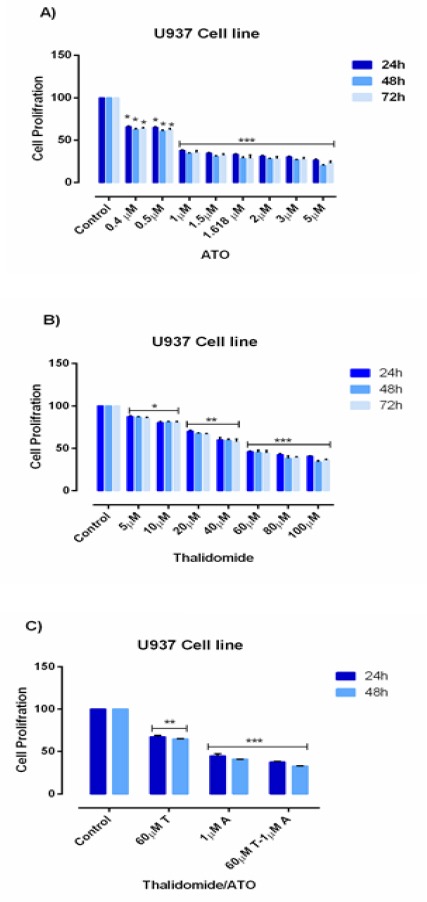
Cell Proliferation in U937. Effects of ATO and THAL on cell proliferation of U937 cell lines. The anti-proliferative effects of ATO (0-5 μM), THAL (0-100 μM) and their combinations on U937 were assessed by MTT assay after 24 h-48h and 72 h treatment. Significant difference between 48h and 72h was not observed. After detection of suitable doses for ATO (1μM) and Thalidomide (60μM), we evaluated combination effect of ATO and THAL. Combination effect of ATO and THAL compared to the control or even single compound could significantly decrease cell proliferation in U937 cell lines. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Figure 5.
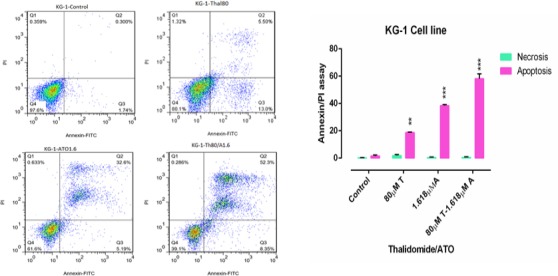
Flow Cytometry of KG-1 Cells Treated with ATO (1.618 µM) and THAL (80µM) and Their Combination. The lower left quadrant shows live cells; the lower right, early apoptotic cells; the upper right, late apoptotic cells and the upper left quadrant shows necrotic cells. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Figure 6.
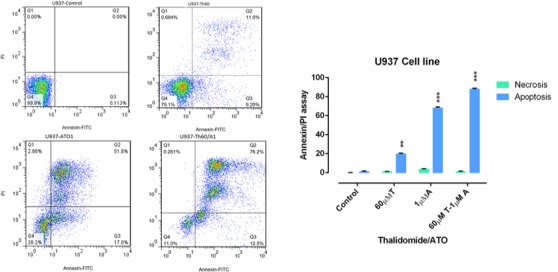
Flow Cytometry of U937 Cells Treated with ATO (1µM) and THAL (60µM) and Their Combination. The lower left quadrant shows live cells; the lower right, early apoptotic cells; the upper right, late apoptotic cells and the upper left quadrant shows necrotic cells. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Apoptosis
Flow cytometry was performed to assess the effect of cited compound on apoptosis induction. Annexin-V/PI staining indicated that ATO and THAL induced the apoptosis. According to the obtained data, we observed significant increase in percentage of early apoptotic cells and minimal percentage of necrotic as compared with the control. In addition, significant increase of apoptotic cells (61 % in KG-1 and 88 % in U937) were seen in combination of ATO and THAL.
Cell Cycle analysis
DNA content of KG-1 and U937 cells assessed after treatment with cited compounds and their combination by PI staining. According to the cell cycle analysis we observed G1 area increased in KG-1 and U937 cells when treated with ATO and THAL and their combination. The percentages of cells at G2 phase was simultaneously reduced in all treated cells. Therefore, it seems that combination of ATO and THAL induce sub G1/G1 arrest (5.71% to 16.32% for KG-1 cell and 5.05% to 36.87% for U937) in both cell lines (Figure 7A and 7B).
Figure 7.
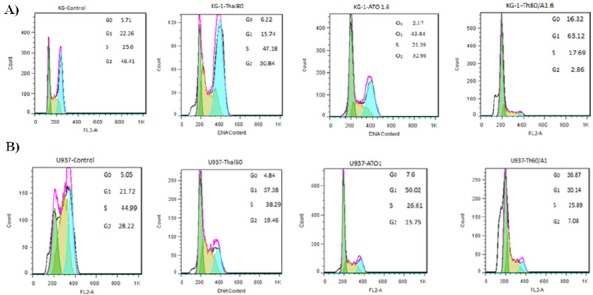
Cell Cycle Flow Cytometry Analysis of Leukemia Cells. 48h exposure to different concentrations of ATO and THAL reduces the number of cells at G2 phase and increases the amount of cells at G1 phase of the cell cycle in KG-1, and U937 cells and was observed significant accumulation of cells in theG0/G1 phase.. Statistical significance were defined at * p <0.05, ** p <0.01 and *** p <0.001 compared to corresponding control.
Real-Time PCR assay
KG-1 and U937 cells were treated with certain concentrations of ATO and THAL for 48h, and they were then examined for expression of VEGFA, VEGFB, VEGFC, VEGFD by the Real-Time PCR. According to these results, the expression level of VEGFA and VEGFB was significantly lower in treated cells than the untreated cells with maximum effect at the concentrations of 80 µM and 60µM of THAL on KG-1 and U937 cell lines and 1.618µM and 1 µM of ATO on KG-1 and U937 respectively (Figures 8A and 8B).
Figure 8.
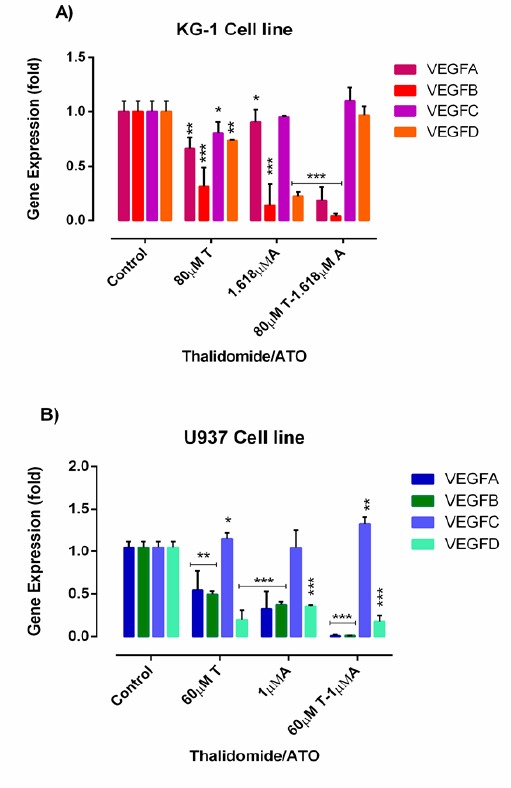
The Effect of THAL and ATO on the Expression Levels of VEGFA, VEGFB, VEGFC, VEGFD was Determined by qRT-PCR Analysis in KG-1(A) and U937 (B) Cells. Values are given as mean ± S.E. of three independent experiments. Statistical significance was defined at *P<0.05, **P<0.01 and ***P<0.001 compared to corresponding untreated controls.
Discussion
AML is a type of cancer which is characterized by the infiltration of blasts to the bone marrow, blood, and other tissues (Döhner et al., 2015; Mohammadi et al., 2017c; Zahed Panah et al., 2017). VEGF is one of the critical regulators of angiogenesis and it affects the proliferation of both solid tumours and haematological malignancies (Rodriguez-Ariza et al., 2011). In the present study, we investigated the effects of ATO and THAL on expression of VEGFA, VEGFB, VEGFC and VEGFD in the leukemic cell lines. AML blast cells cause aberrant expression of VEGF ligands and receptors. The higher VEGF ligand expression is significantly associated with a poor outcome in AML patients. AML blasts enhance the autocrine VEGF signalling in order to increase the proliferation and provide a survival advantage. From therapeutic aspects, VEGF is a well-known target for treatment of AML. VEGF-targeted therapy in the AML patients could inhibit the autocrine VEGF signalling in AML cells as well as the aberrant vessel formation by the vascular endothelial cells (Kampen et al., 2013).
ATO prevents the cell proliferation and induces apoptosis in some cancer cells including the solid tumors and acute promyelocytic leukemia. ATO affects different signaling pathways. Study on the mechanisms of ATO may be useful in designing more effective cancer therapies (Siu et al., 2002; Yu et al., 2007; Morales et al., 2008; Liu et al., 2012).
THAL is an immunomodulatory agent, it can be used as a potential compound for treatment of malignant and immunological disorders. This agent inhibits the angiogenesis by blocking basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF)(Kruse et al., 1998), modulates various cytokines (Corral et al., 1999), enhances cell-mediated immunity through direct co-stimulated T-cells (Haslett et al., 1998), and alters adhesion molecule expression (Geitz et al., 1996).
The present research investigated the cytotoxic effects of ATO and THAL and also the induction of apoptosis in both U937 and KG-1 cell lines. Results of the flow cytometry indicated that these selected doses could induce a significant percentage of apoptosis. Besides, the previous studies indicated the concentration range of 0.5-2 µM (Zhang et al., 1998; Song et al., 2012; Noguera et al., 2017) for induction apoptosis by ATO; hence, the selected single doses of ATO were 1.618µM and 1µM for KG-1 and U937 respectively.
Cell cycle regulation is typically damaged in leukemic cells. ATO induces the cell cycle arrest at G1 in leukemic cells (Noguera et al., 2017). THAL also induces the apoptosis and cell cycle arrest at G1 phase in leukemic cells (Steins et al., 2002). According to the above-mentioned studies, ATO and THAL arrested cell cycle at Sub G1/G1 in both cell lines. According to the previous researches, ATO induces the apoptosis in leukemic cells and blood vessel endothelial cells by a time/dose-dependent manner via inhibition of the VEGFA production (Ge et al., 2015). THAL and other immunomodulatory agents (e.g. lenalidomide) were studied in multiple myeloma which showed a significant decreased in expression of pro-angiogenic factor VEGF and interleukin-6 (IL-6) (Gupta et al., 2001). Real-time data analysis indicated that ATO and THAL down-regulated gene expression of VEGFA, VEGFB, VEGFD and up-regulated the VEGFC in both cell lines. In consistent with our results, THAL induced anti-angiogenesis by at lower levels of FGF-2 and VEGF (Raza et al., 2008), Roboz et al., (2000) found that ATO induced apoptosis in leukemia by inhibiting VEGF. Yang et al., (2014) on the lung cancer model found that the ATO inhibited factors such as VEGF-A, VEGFR-2, HIF-1α and Notch-1, angiogenesis pathway (Yang et al., 2014).
In conclusion, to sum up, THAL as a VEGF inhibitor in combination with ATO has a synergistic impact on the inhibition of cell proliferation and promotion of apoptosis in AML cell lines. ATO enhances the anti-leukemic activity of THAL in both U937 and KG-1 cell populations when it is simultaneously used in combination.
Acknowledgments
The present study was conducted and funded by The Hematology-Oncology and Stem cell Transplantation Institute, Tehran University of Medical Sciences.
References
- 1.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163:380–6. [PubMed] [Google Scholar]
- 2.D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 4.Drake M, Robson W, Mehta P, et al. An open-label phase II study of low-dose thalidomide in androgen-independent prostate cancer. Br J Cancer. 2003;88:822. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen T, Boshoff C, Vaughan M, et al. Anti-angiogenic treatment of metastatic melanoma, renal cell, ovarian and breast cancers with thalidomide:a phase II study. Proc Am Soc Clin Oncol. 1998;1998:441a. [Google Scholar]
- 6.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Res. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 7.Figg W, Bergan R, Brawley O, et al. Randomized phase II study of thalidomide in androgen-independent prostate cancer (AIPC) Proc Am Soc Clin Oncol. 1997:333a. [Google Scholar]
- 8.Ge H-y, Han Z-j, Tian P, et al. VEGFA expression is inhibited by arsenic trioxide in HUVECs through the upregulation of Ets-2 and miRNA-126. PLoS One. 2015;10:e0135795. doi: 10.1371/journal.pone.0135795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–21. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta D, Treon S, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion:therapeutic applications. Leukemia. 2001;15:1950. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 11.Haghi A, Mohammadi S, Heshmati M, et al. Anti-vascular endothelial growth factor effects of sorafenib and arsenic trioxide in acute myeloid leukemia cell lines. Asian Pac J Cancer Prev. 2017;18:1655–61. doi: 10.22034/APJCP.2017.18.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haslett PA, Corral LG, Albert M, et al. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+subset. J Exp Med. 1998;187:1885–92. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampen KR, Ter Elst A, de Bont ES. Vascular endothelial growth factor signaling in acute myeloid leukemia. Cell Mol Life Sci. 2013;70:1307–17. doi: 10.1007/s00018-012-1085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenyon BM, Browne F, D'amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–8. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 15.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse FE, Joussen AM, Rohrschneider K, et al. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch Clin Exp Ophthalmol. 1998;236:461–6. doi: 10.1007/s004170050106. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Jiang A, Pei D. Identification and characterization of a new splicing variant of vascular endothelial growth factor:VEGF1831. Biochim Biophys Acta. 1998;1443:400–6. doi: 10.1016/s0167-4781(98)00240-1. [DOI] [PubMed] [Google Scholar]
- 18.Lew YS, Brown SL, Griffin RJ, et al. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999;59:6033–7. [PubMed] [Google Scholar]
- 19.Liu W, Gong Y, Li H, et al. Arsenic trioxide-induced growth arrest of breast cancer MCF-7 cells involving FOXO3a and IκB kinase βexpression and localization. Cancer Biother Radiopharm. 2012;27:504–12. doi: 10.1089/cbr.2012.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long G, Vredenburgh J, Rizzieri D. Pilot trial of thalidomide post-autologous peripheral blood progenitor cell transplantation (PBPC) in patients with metastatic breast cancer. Proc Am Soc Clin Oncol. 1998;1998:181a. [Google Scholar]
- 21.Marx G, Levi J, Bell D, et al. A phase I/II trial of thalidomide as an antiangiogenic agent in the treatment of advanced cancer. Proc Annu Meet Am Soc Clin Oncol. 1999;1999:454. [Google Scholar]
- 22.Miller WH, Schipper HM, Lee JS, et al. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903. [PubMed] [Google Scholar]
- 23.Mirzaei A, Ghaffari SH, Nikbakht M, et al. OPN b and c isoforms doubtless veto anti-angiogenesis effects of curcumin in combination with conventional AML regiment. Asian Pac J Cancer Prev. 2017;18:2591–9. doi: 10.22034/APJCP.2017.18.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadi S, Ghaffari SH, Shaiegan M, et al. Acquired expression of osteopontin selectively promotes enrichment of leukemia stem cells through AKT/mTOR/PTEN/β-catenin pathways in AML cells. Life Sci. 2016;152:190–8. doi: 10.1016/j.lfs.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi S, Nikbakht M, Sajjadi SM, et al. Reciprocal interactions of leukemic cells with bone marrow stromal cells promote enrichment of leukemic stem cell compartments in response to curcumin and daunorubicin. Asian Pac J Cancer Prev. 2017a;18:831–40. doi: 10.22034/APJCP.2017.18.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammadi S, Zahedpanah M, Ghaffari SH, et al. Osteopontin plays a unique role in resistance of CD34+/CD123+human leukemia cell lines KG1a to parthenolide. Life Sci. 2017b;189:89–95. doi: 10.1016/j.lfs.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadi S, Zahedpanah M, Nikbakht M, et al. Parthenolide reduces gene transcription of prosurvival mediators in U937 cells. Exp Oncol. 2017c;39:30–5. [PubMed] [Google Scholar]
- 28.Morales AA, Gutman D, Lee KP, et al. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide–induced cell death in myeloma. Blood. 2008;111:5152–62. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguera NI, Pelosi E, Angelini DF, et al. High-dose ascorbate and arsenic trioxide selectively kill acute myeloid leukemia and acute promyelocytic leukemia blasts in vitro. Oncotarget. 2017;8:32550. doi: 10.18632/oncotarget.15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells:novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 31.Raza A, Mehdi M, Mumtaz M, et al. Combination of 5-azacytidine and thalidomide for the treatment of myelodysplastic syndromes and acute myeloid leukemia. Cancer. 2008;113:1596–604. doi: 10.1002/cncr.23789. [DOI] [PubMed] [Google Scholar]
- 32.Roboz GJ, Dias S, Lam G, et al. Arsenic trioxide induces dose-and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–30. [PubMed] [Google Scholar]
- 33.Rodriguez-Ariza A, Lopez-Pedrera C, Aranda E, et al. VEGF targeted therapy in acute myeloid leukemia. Crit Rev Oncol Hematol. 2011;80:241–56. doi: 10.1016/j.critrevonc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Salemi M, Ghavamzadeh A, Nikbakht M. Anti-vascular endothelial growth factor targeting by curcumin and thalidomide in acute myeloid leukemia cells. Asian Pac J Cancer Prev. 2017;18:3055–61. doi: 10.22034/APJCP.2017.18.11.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu KP, Chan JY, Fung K. Effect of arsenic trioxide on human hepatocellular carcinoma HepG2 cells:inhibition of proliferation and induction of apoptosis. Life Sci. 2002;71:275–85. doi: 10.1016/s0024-3205(02)01622-3. [DOI] [PubMed] [Google Scholar]
- 36.Song G, Li Y, Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets. Oncol Rep. 2012;28:1935–44. doi: 10.3892/or.2012.2045. [DOI] [PubMed] [Google Scholar]
- 37.Steins MB, Padró T, Bieker R, et al. Efficacy and safety of thalidomide in patients with acute myeloid leukemia. Blood. 2002;99:834–9. doi: 10.1182/blood.v99.3.834. [DOI] [PubMed] [Google Scholar]
- 38.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 39.Woodyatt P. Thalidomide. Lancet. 1962;279:750. [Google Scholar]
- 40.Yang M-H, Zang Y-S, Huang H, et al. Arsenic trioxide exerts anti-lung cancer activity by inhibiting angiogenesis. Curr Cancer Drug. 2014;14:557–66. doi: 10.2174/1568009614666140725090000. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Qian H, Li Y, et al. Arsenic trioxide (As2O3) reduces the invasive and metastatic properties of cervical cancer cells in vitro and in vivo. Gynecol Oncol. 2007;106:400–6. doi: 10.1016/j.ygyno.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Zahed Panah M, Nikbakht M, Sajjadi SM, et al. Anti-apoptotic effects of osteopontin via the up-regulation of AKT/mTOR/beta-Catenin loop in acute myeloid leukemia cells. Int J Hematol Oncol Stem Cell Res. 2017;11:148–57. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Ohnishi K, Shigeno K, et al. The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms. Leukemia. 1998;12:1383. doi: 10.1038/sj.leu.2401112. [DOI] [PubMed] [Google Scholar]


