Abstract
Background and Purpose
Hand tremor is one of the most frequent symptoms in movement disorders, and differential diagnoses for hand tremor include Parkinson's disease (PD) and essential tremor (ET). However, accurately differentiating between PD and ET in clinical practice remains challenging in patients presenting with hand tremor. We investigated whether a questionnaire-based survey could be useful as a screening tool in patients with hand tremor.
Methods
A questionnaire related to hand tremor consisting of 12 items was prospectively applied to patients with PD or ET in three movement-disorder clinics. Each question was analyzed, and a query-based scoring system was evaluated for differentiating hand tremors between PD and ET.
Results
This study enrolled 24 patients with PD and 25 patients with ET. Nine of the 12 questions differed significantly between PD and ET: 1 about resting tremor, 4 questions about action tremor, and 4 about asymmetry. A receiver operating characteristics curve analysis revealed that the 9-item questionnaire showed a good discrimination ability, with a sensitivity of 88% and a specificity of 84%.
Conclusions
The developed Hand Tremor Questionnaire might be a good screening tool for hand tremors in patients with PD and ET.
Keywords: tremor, Hand Tremor Questionnaire, screening, Parkinson's disease, essential tremor, hand
INTRODUCTION
Common movement disorders that present with hand tremor include Parkinson's disease (PD) and essential tremor (ET). Patients with PD exhibit hand tremors predominantly in the resting state, while patients with ET exhibit hand tremors when active. However, physicians sometimes have difficulty discriminating between PD and ET due to various types of hand tremors such as resting or kinetic tremor being observed in patients with either PD or ET. About 90% of patients with PD reportedly present with action tremors, while 2–47% patients with ET show resting tremors in their hands.1,2,3,4,5 Moreover, many patients with ET exhibit asymmetric hand tremors,4,6,7 even though asymmetry of hand tremor is considered a typical feature of PD.8 Accordingly, 37–50% of patients with ET are misdiagnosed, and in most of them the correct diagnosis would be PD.8,9,10
Many previous electrophysiological studies have used myography-based or accelerometry-based tremography to investigate the differences in the characteristics of the hand tremors between patients with PD and ET. However, the measured values of tremor parameters have not been consistent, including in their asymmetry, intensity, frequency, and muscle contraction pattern. 4,7,11,12,13,14,15 Positron-emission tomography (PET) with a radioactive isotope has recently been used to evaluate brain function in vivo in a study of neurodegenerative disorders including PD.16 PET scans for dopamine transporter (DAT) have enabled clinicians to more accurately distinguish PD from ET.17,18 DAT imaging is widely accepted as a gold-standard test for distinguishing PD from other nonneurodegenerative disorders, including ET.19,20 However, such an expensive neuroimaging technique can only be applied in a small number of well-equipped hospitals, such as university/tertiary hospitals.
A less-expensive but accurate tool that is feasible to use to discriminate hand tremors between patients with PD and ET would be useful for primary physicians who evaluate patients presenting with hand tremors. To the best of our knowledge, questionnaires about hand tremors have not been used previously to differentiate between PD and ET. We therefore aimed to determine the questions about hand tremor that would be useful for distinguishing between PD and ET. We addressed this by developing the Hand Tremor Questionnaire and testing it on patients with previously diagnosed PD or ET. The questionnaire was then further evaluated to determine discriminative values for utilizing it as a screening tool.
METHODS
Development of the Hand Tremor Questionnaire
A task-force team consisting of the following movement-disorder specialists was assembled to develop the Hand Tremor Questionnaire: K-Y.K., H-S.R., M-J.K., H-W.S., H.K.P., S. J.C., and S-B.K. We weighted and selected questions associated with favorable responses for both PD and ET based on our clinical experience. After thorough discussions, we selected five questions (Q1–Q5) for PD (i.e., to which patients with PD might answer ‘yes’) and seven questions (Q6–Q12) for ET (i.e., to which patients with ET might answer ‘yes’). The questionnaire therefore consisted of two parts: Q1–Q5 were related to PD, while Q6–Q12 were related to ET. We also considered the possibility that some patients would not choose to answer ‘yes’ or ‘no’ to some questions because they might not recognize their hand tremors in certain situations, and so ‘uncertain’ was added as a response choice for each question. Supplementary Table 1 (in the online-only Data Supplement) presents the final version of the developed questionnaire.
Participants and study protocol
This study was prospectively designed as a cross-sectional, questionnaire-based research study at three movement-disorder clinics in South Korea, and it was approved by each Institutional Review Board at Korea University Guro Hospital (IRB No. KUGH16029), Asan Medical Center (IRB No. S2016-0330-0004), and Soonchunhyang University Hospital (IRB No. SCH2016-06). We enrolled consecutive patients with PD or ET between April and December 2016. Written informed consent was obtained from each participant before their enrollment. Neurological examinations were performed on all participants to exclude atypical or uncertain diagnoses, including PD combined with ET. PD was diagnosed in accordance with the UK brain bank criteria,8 and ET was diagnosed using the Movement Disorder Society consensus criteria.21 Furthermore, only subjects with illness durations exceeding 3 years were enrolled to rule out atypical parkinsonism and increase the accuracy of the clinical diagnoses. Because this study focused on hand tremors, patients with PD without hand tremors were excluded. Patients who had psychiatric illnesses or moderate-to-severe dementia were also excluded from the study.
All of the subjects assigned to the study were asked to complete the Hand Tremor Questionnaire. While the patients were being treated with their routine medications, motor examinations were performed again to confirm the clinical diagnoses. Patients with PD were assessed using all of Part III (motor) of the Unified Parkinson's Disease Rating Scale (UPDRS-III), whereas the patients with ET were assessed using only the tremor subscale of UPDRS-III. This procedure did not result in any alteration of the clinical diagnoses being altered.
Statistical analyses
While response choices were available for each question in the Hand Tremor Questionnaire, when analyzing the data we recategorized those answers into two variables (‘yes’ vs. ‘no’/‘uncertain’). Group differences between the patients with PD and ET were assessing using Mann-Whitney U tests for continuous or ordinal variables after testing for normality, and the chi-square test or Fisher's exact tests for categorical variables. Probability values of p<0.05 indicated statistical significance. Receiver operating characteristics curve analyses were performed to obtain the discriminative power for the diagnosis of PD or ET. The optimal cutoff was determined using the Youden index method. This calculation system means that higher scores are associated with a higher probability of having PD. All analyses were conducted using SPSS for Windows (version 20.0, IBM Corp., Armonk, NY, USA).
RESULTS
Characteristics of patients with Parkinson's disease and essential tremor
A total of 49 subjects (24 patients with PD and 25 patients with ET) participated in the study. Nine of them had previously undergone PET scans for DAT to confirm their clinical diagnoses at the time of their initial diagnoses. The demographic and clinical characteristics of the patients are presented in Table 1. Compared with patients with PD, patients with ET were younger and had longer disease durations; however, the family history did not differ between the disorders. As expected, patients with PD exhibited rest-tremor-dominant features, while patients with ET exhibited action-tremor-dominant features in their neurological examinations.
Table 1. Demographic and clinical characteristics in patients with PD and ET.
| Characteristics | PD (n=24) | ET (n=25) | Statistics | |
|---|---|---|---|---|
| Z or χ2 | p | |||
| Females | 12 (50) | 14 (56) | 0.177 | 0.674† |
| Age, years | 70.8±7.6 | 57.4±18.2 | −3.055 | 0.002 |
| Education duration, years | 8.3±5.4 | 7.8±5.7 | −0.357 | 0.747 |
| Disease duration, years | 5.1±2.4 | 12.5±8.5 | −3.796 | <0.001 |
| Handedness, right/left or both | 18 (75)/6 (25) | 24 (96)/1 (4) | n.a. | 0.036* |
| Family history, yes/no or uncertain | 4 (16.7)/20 (83.3) | 6 (24)/19 (76) | n.a. | 0.524* |
| UPDRS-III score | 20.3±11.3 | n.a. | n.a. | n.a. |
| Tremor score in both hands at rest | 2.3±1.3 | 0.0±0.2 | −5.753 | <0.001 |
| Resting tremor score in more-affected hand | 1.8±0.8 | 0.1±0.4 | −5.825 | <0.001 |
| Resting tremor score in less-affected hand | 0.6±0.4 | 0.0±0.2 | −3.617 | 0.001 |
| Tremor score in both hands during activity/posture | 1.4±1.4 | 2.4±1.3 | −2.670 | 0.008 |
| Kinetic tremor score in more-affected hand | 0.9±0.8 | 1.5±0.8 | −2.660 | 0.008 |
| Kinetic tremor score in less-affected hand | 0.5±0.7 | 1.0±0.7 | −2.235 | 0.025 |
| Hoehn & Yahr stage | 2.1±0.2 | n.a. | n.a. | n.a. |
Data are mean±standard-deviation or n (%) values. Continuous or ordinal variables were evaluated only using the Mann-Whitney U test with the Z value after testing for normality. For categorical variables. p values were assessed using.
*Fisher's exact test or the †Chi-square test.
ET: essential tremor, n.a.: not applicable, PD: Parkinson's disease, UPDRS-III: Part III of the Unified Parkinson's Disease Rating Scale.
Detailed results from the Hand Tremor Questionnaire
Before conducting the survey with the Hand Tremor Questionnaire we considered that all of the questions might be important in differentiating between PD and ET. However, some questions were found not to be; the detailed data are presented in Table 2. Only three of the five questions focused on PD were found to be valuable. In particular, only one (Q1) of the three questions (Q1–Q3) associated with resting tremor showed a significant difference. In contrast, six (Q6–Q11) of the seven questions focused on ET showed clinically meaningful differences; the exception was the question related to alcohol responsiveness. The four questions (Q8–Q11) associated with action tremor were all important in differentiating between the two disorders. Regarding asymmetry of the hand tremor, patients with PD showed an asymmetric onset (Q4) and current asymmetric feature (Q5) of tremor, and vice versa in patients with ET (Q6 and Q7).
Table 2. Hand Tremor Questionnaire: results for each question in differentiating between PD (n=24) and ET (n=25).
| Question | PD | ET | p |
|---|---|---|---|
| 1 Does your hand tremor worsen when you relax your arms? | 15 (62.5) | 4 (16) | 0.001* |
| 2 Does your hand tremor worsen while focusing on something? (e.g., watching TV or talking) | 6 (25) | 7 (28) | 0.812† |
| 3 Does your hand tremor worsen (e.g., you can't feel it or realize it is there) when someone looks at you? | 13 (54.2) | 15 (60) | 0.680† |
| 4 Does your tremor in one hand worsen more than in the other hand, or does only one hand show tremor? | 24 (100) | 12 (48) | <0.001* |
| 5 Did your hand tremor start to develop on one side only? | 24 (100) | 11 (44) | <0.001* |
| 6 Did your tremor in both hands start to develop at similar times? | 0 (0) | 13 (52) | <0.001* |
| 7 Currently, are both of your hands shaking similarly? | 0 (0) | 13 (52) | <0.001* |
| 8 Does your hand tremor worsen when holding something or stretching your arm? | 6 (25) | 15 (60) | 0.013† |
| 9 Does your hand tremor worsen when using chopsticks or a spoon? | 6 (25) | 18 (72) | 0.001† |
| 10 Does your hand tremor worsen when you write letters? | 4 (16.7) | 18 (72) | <0.001* |
| 11 Does your hand tremor worsen when holding something? (e.g., a cup or cellular phone) | 9 (37.5) | 21 (84) | 0.001* |
| 12 Does your hand tremor reduce when drinking alcohol, or worsen when sobering up after drinking alcohol? | 4 (16.7) | 9 (36) | 0.196* |
Data are n (%) values for ‘yes’ responses. p values were assessed using.
*Fisher's exact test or the †Chi-square test.
ET: essential tremor, PD: Parkinson's disease.
Receiver operating characteristics curve analysis and discriminative values for differentiating Parkinson's disease and essential tremor
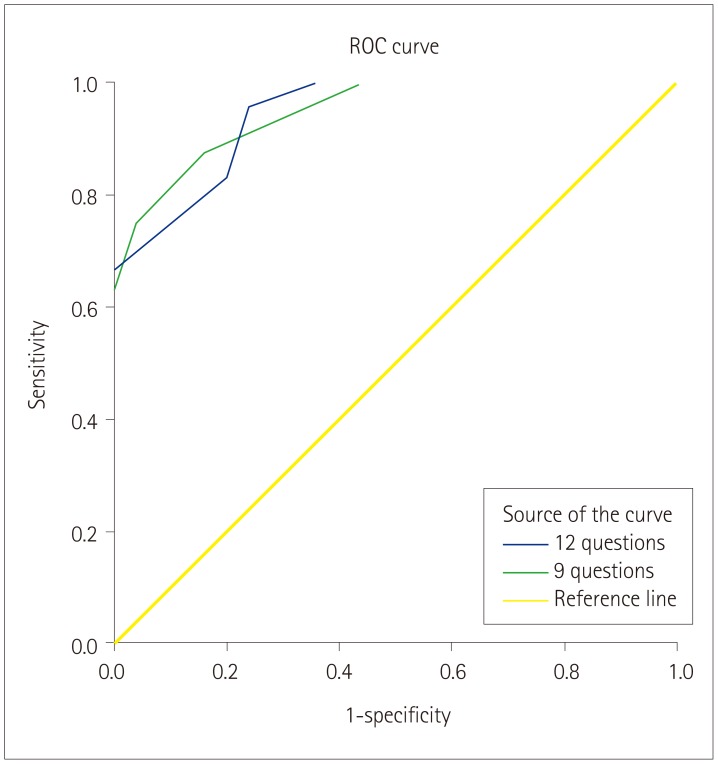
After detailed analyses of each question on hand tremor, we further investigated the questions to obtain discriminative values to use in a screening tool for differentiating the two disorders. Specifically, a ‘yes’ response was given a score of 1 point in Q1–Q5, while ‘no’/‘uncertain’ responses were counted as 1 point in Q6–Q12. Furthermore, because three questions (Q2, Q3, and Q12) were not significant in the Hand Tremor Questionnaire, we performed the analysis with 2 questionnaires: the first comprising 12 items (from the initial questionnaire) and the second comprising only the 9 significant items (Table 3, Fig. 1). After comparing the 2 questionnaires, we concluded that the 9-item one might be more appropriate for differentiating between PD and ET. The optimal cutoff score for distinguishing PD from ET was 5.5 points in the 9-item questionnaire, which yielded an area under the receiver operating characteristics curve of 0.947, a sensitivity of 0.875, and a specificity of 0.84.
Table 3. Clinical usefulness of the Hand Tremor Questionnaire in the differentiating between PD and ET.
| Cutoff score | Sensitivity (%) | Specificity (%) | AUC (%) | |
|---|---|---|---|---|
| 12 questions | 6.5 | 95.8 | 76 | 94.3 |
| 9 questions | 5.5 | 87.5 | 84 | 94.7 |
Total scores equal to or higher than the cutoff might indicate PD, whereas lower scores might indicate ET.
AUC: area under the receiver operating characteristics curve, ET: essential tremor, PD: Parkinson's disease.
Fig. 1. ROC curve analysis to discriminate between Parkinson's disease and essential tremor: using 12 versus 9 questions. ROC: receiver operating characteristics.
DISCUSSION
We have demonstrated that it might be possible to use a questionnaire-based survey of hand tremors to discriminate between PD and ET. Furthermore, we suggest that the developed Hand Tremor Questionnaire could be useful as a screening tool in patients with hand tremor. PD and ET are clinically diagnosed on the basis of neurological examinations. Accordingly, the critical roles of the neurologist are not only to diagnose but also treat the two disorders. However, the importance and usefulness of clinical history in hand tremors remain underestimated. In this study we evaluated the real feelings or sensations of the hand tremors experienced in the daily lives of patients with PD or ET. Table 2 in the Hand Tremor Questionnaire indicates that 9 out of 12 questions revealed useful differences. These results suggest that taking a detailed history might be very helpful in diagnosing a patient with hand tremor. Our study highlights that physicians should pay attention to the complaints of patients about tremors. Furthermore, our questionnaire might also provide the general public with valuable information, since many of the subjects with hand tremors who are very concerned about the possibility of PD before seeing a doctor will actually have ET.
A reliable screening questionnaire for people with hand tremors could help physicians to more accurately differentiate between PD and ET. We aimed to design this questionnaire as a screening tool for discriminating the two disorders. Table 3 indicates that the 9-item questionnaire produced a sensitivity of 88% and a specificity of 84%, thus demonstrating its usefulness as a diagnostic tool for differentiating between PD and ET. Physicians utilizing our simple questionnaire as a screening tool could improve the diagnostic accuracy in people with hand tremors.
Three questions (Q1–Q3) were initially designed to target resting hand tremors for subjects with PD or ET, as listed in Table 2. Unexpectedly, only one of these questions (Q1) showed a meaningful difference. We deduced that Q1 might reflect the true resting state, with both Q2 and Q3 not fully representing the resting state. A person who recognizes his or her own tremor while watching TV or talking could naturally become nervous or anxious. Likewise, someone with tremor is frequently nervous about this condition being perceived by others. Accordingly, clinicians need to be cautious when interpreting the clinical history when assessing resting tremor. It is reasonable to infer that resting tremor could be more easily observed by the caregiver or physician than by the patient. On the other hand, action tremor was surveyed through four of the questions in Table 2 (Q8 and Q11 for postural tremor, and Q9 and Q10 for kinetic tremor), and we found that all four questions were significant in discriminating between PD and ET. This suggests that, in contrast to a resting tremor, any complaint of an action tremor might be a diagnostic clue in patients presenting with hand tremor. Together these observations suggest that resting tremor is more important when examining a patient, while action tremor is more important when obtaining the clinical history from a patient.
Most studies have assessed tremor asymmetry not only through neurological examinations but also by using objective tools, including tremography. However, the clinical significance of asymmetry of the hand tremor from the perspective of the patient remains unclear. When first developing the questionnaire we did not know which question focused on tremor asymmetry/symmetry would have a discriminative value. Accordingly, we prepared two different questions (one for an asymmetric onset and one for current asymmetry) for PD (Q4 and Q5, respectively) and ET (Q6 and Q7, respectively). We found that both the onset and current features of hand tremor asymmetry/symmetry were clinically significant in both disorders. Considering our inclusion criterion of the disease duration being ≥3 years, this may indicate that such characteristics of hand tremor are useful for discriminating between the two disorders irrespective of the assessment time.
An improvement in tremor after alcohol intake is generally thought to be associated with ET and not with PD, although other movement disorders also show alcohol responsiveness.22 Unexpectedly, our study revealed that the responses to the question on alcohol (Q12) (Table 2) did not differ significantly between patients with PD and ET. Some patients (16.7%) with PD as well as a considerable proportion of the patients (36%) with ET answered that their tremors were relieved when drinking alcohol (p=0.125). In line with our observation, a previous study found that alcohol intake could improve action tremors in patients with PD.23 However, the exact mechanism underlying alcohol-responsive tremor in PD has not been investigated, while several mechanisms have been studied in ET.22
We acknowledge that the current study had some limitations. First, the tremor characteristics identified using the questionnaire-based survey were not confirmed with clinicians or caregivers. In addition, more-precise measurements for resting and action tremors were not assessed, and voice- or head-tremor-associated questions were not included in the questionnaire. We focused on the perspectives that the patients themselves had about hand tremor under several different conditions, and so the objective characteristics of hand tremor as revealed by tremography might differ from the results of the present questionnaire-based survey. In clinical practice, clinicians occasionally find that patients presenting with hand tremors distort their tremors. Collectively, the results of our questionnaire-based survey might not represent objective findings, instead being the subjective assessments of patients with PD or ET. Nonetheless, the 9-item questionnaire showed good sensitivity and specificity (Table 3), indicating that it is a useful candidate screening method. Second, the sample of participants was relatively small. However, the study was performed in three university hospitals where all physicians were experienced movement-disorder specialists. In addition, this study has provided preliminary evidence that a questionnaire is useful in distinguishing PD and ET. Third, this was an exploratory study of screening hand tremors in clinically diagnosed patients with PD or ET using a questionnaire. We could not include patients in the early stages of the two disorders because one of the inclusion criteria was a disease duration of ≥3 years. Fourth, only some participants with severe tremor were recruited in the current study, and severe cases with PD or ET could show both resting and action tremors. Accordingly, this study might not reflect the wide range of hand tremors experienced by patients with PD or ET. Therefore, a validation study of the questionnaire is warranted to generalize our questionnaire as a screening tool.
In conclusion, the developed Hand Tremor Questionnaire might be not only useful but also feasible to apply to patients with hand tremors who present in real clinical practices.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.3.381.
Hand Tremor Questionnaire
References
- 1.Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–410. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Hernandez N, Michalec M. Prevalence and correlates of rest tremor in essential tremor: cross-sectional survey of 831 patients across four distinct cohorts. Eur J Neurol. 2015;22:927–932. doi: 10.1111/ene.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Asabere N, Agnew A, Moskowitz CB, Lawton A, Cortes E, et al. Rest tremor in advanced essential tremor: a post-mortem study of nine cases. J Neurol Neurosurg Psychiatry. 2011;82:261–265. doi: 10.1136/jnnp.2010.215681. [DOI] [PubMed] [Google Scholar]
- 4.Kwon KY, Lee HM, Lee SM, Kang SH, Koh SB. Comparison of motor and non-motor features between essential tremor and tremor dominant Parkinson's disease. J Neurol Sci. 2016;361:34–38. doi: 10.1016/j.jns.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann R, Deuschl G, Hornig A, Schulte-Mönting J, Fuchs G, Lücking CH. Tremors in Parkinson's disease: symptom analysis and rating. Clin Neuropharmacol. 1994;17:303–314. [PubMed] [Google Scholar]
- 6.Louis ED, Wendt KJ, Pullman SL, Ford B. Is essential tremor symmetric? observational data from a community-based study of essential tremor? Arch Neurol. 1998;55:1553–1559. doi: 10.1001/archneur.55.12.1553. [DOI] [PubMed] [Google Scholar]
- 7.Farkas Z, Csillik A, Szirmai I, Kamondi A. Asymmetry of tremor intensity and frequency in Parkinson's disease and essential tremor. Parkinsonism Relat Disord. 2006;12:49–55. doi: 10.1016/j.parkreldis.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 10.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor--some controversial aspects. Mov Disord. 2011;26:18–23. doi: 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
- 11.Machowska-Majchrzak A, Pierzchała K, Pietraszek S. Analysis of selected parameters of tremor recorded by a biaxial accelerometer in patients with parkinsonian tremor, essential tremor and cerebellar tremor. Neurol Neurochir Pol. 2007;41:241–250. [PubMed] [Google Scholar]
- 12.Cichaczewski E, Munhoz RP, Maia JM, Nohama P, Nóvak EM, Teive HA. Electrophysiologic characteristics of tremor in Parkinson's disease and essential tremor. Arq Neuropsiquiatr. 2014;72:301–306. doi: 10.1590/0004-282x20140006. [DOI] [PubMed] [Google Scholar]
- 13.Burne JA, Hayes MW, Fung VS, Yiannikas C, Boljevac D. The contribution of tremor studies to diagnosis of parkinsonian and essential tremor: a statistical evaluation. J Clin Neurosci. 2002;9:237–242. doi: 10.1054/jocn.2001.1017. [DOI] [PubMed] [Google Scholar]
- 14.Breit S, Spieker S, Schulz JB, Gasser T. Long-term EMG recordings differentiate between parkinsonian and essential tremor. J Neurol. 2008;255:103–111. doi: 10.1007/s00415-008-0712-2. [DOI] [PubMed] [Google Scholar]
- 15.Nisticò R, Pirritano D, Salsone M, Novellino F, Del Giudice F, Morelli M, et al. Synchronous pattern distinguishes resting tremor associated with essential tremor from rest tremor of Parkinson's disease. Parkinsonism Relat Disord. 2011;17:30–33. doi: 10.1016/j.parkreldis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Rinne JO. Positron emission tomography in the differential diagnosis of parkinsonism. J Mov Disord. 2009;2:53–57. doi: 10.14802/jmd.09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benamer HTS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. 2000;15:503–510. [PubMed] [Google Scholar]
- 18.Parkinson Study Group. A multicenter assessment of dopamine transporter imaging with DOPASCAN/SPECT in parkinsonism. 2000. Neurology. 2001;57(10 Suppl 3):S52–S59. [PubMed] [Google Scholar]
- 19.Ba F, Martin WR. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. 2015;21:87–94. doi: 10.1016/j.parkreldis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson's disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res. 2015;5:12. doi: 10.1186/s13550-015-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Ad hoc scientific committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 22.Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25:2274–2284. doi: 10.1002/mds.23240. [DOI] [PubMed] [Google Scholar]
- 23.Rajput AH, Jamieson H, Hirsh S, Quraishi A. Relative efficacy of alcohol and propranolol in action tremor. Can J Neurol Sci. 1975;2:31–35. doi: 10.1017/s0317167100019958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hand Tremor Questionnaire