Abstract
Background and Purpose
The objective of this study was to determine the frequencies of different clinical presentations and the phenotypic spectrum of multiple sclerosis (MS).
Methods
This cross-sectional study was performed in the Neurology Department of King Fahd Hospital of University Alkhobar in the Kingdom of Saudi Arabia (KSA). Data of 190 MS patients who fulfilled the McDonald criteria were retrieved from medical records and analyzed.
Results
The age at disease onset was 26.27±8.2 years (mean±SD) and disease duration was 6.38±5.10 years. The male-to-female ratio was 1:1.6. Optic neuritis and myelitis were the most-frequent first clinical presentations. Sensory (73.1%), motor (61%), and visual (58.4%) symptoms were the most-frequent established clinical symptoms. Relapsing-remitting multiple sclerosis (RRMS) was present in 75% of the cases. Supratentorial T2-weighted white-matter lesions and deep-gray-matter or juxtacortical lesions were the most-frequent magnetic resonance imaging (MRI) lesions, comprising 28% and 23.7% of all MRI lesions observed in 93.6% and 79.4% of the cases, respectively. The scores on the Expanded Disability Status Scale were within the range of 1.0–5.5 in 82.1% of the patients. There were 145 (76.3%) patients taking interferon β therapy.
Conclusions
MS presenting in the hospital setting is more common in KSA than reported previously, and the number of diagnosed cases in increasing. It is therefore an emerging and disabling neurological illness in KSA with clinical characteristics not dissimilar to those in other middle eastern countries. A decrease in the frequency of patients with secondary progressive multiple sclerosis (SPMS) indicates either that more new cases of RRMS are being diagnosed or that adequate treatments of RRMS are preventing the evolution to SPMS. Further larger and population-wide epidemiological and clinical studies with the long-term follow-up of MS patients are required to better assess the clinical spectrum of MS in KSA.
Keywords: multiple sclerosis, phenotype, epidemiology, prevalence
INTRODUCTION
Multiple sclerosis (MS) is the most-common immune-mediated demyelinating disease of the central nervous system, with a wide range and heterogeneous clinical presentations with no definitive symptoms, but with certain highly characteristic symptoms and signs such as unilateral subacute visual loss, bilateral internuclear ophthalmoplegia, subcute spinal-cord syndromes, fatigue, and heat sensitivity. Common symptoms and signs of MS in decreasing order of presentation frequency are sensory symptoms in the limbs or face, unilateral visual loss, subacute motor weakness, diplopia, balance problems with gait disturbances, vertigo, limb ataxia, bladder symptoms, and pain.1 The main MS phenotypes are relapsing disease and progressive disease, which are further classified into several clinical subtypes depending on the disease activity (with relapses or new MRI lesions forming over time).2 Typically 85–90% of patients have relapsing-remitting multiple sclerosis (RRMS), with clinically isolated syndrome (CIS) presenting as the first manifestations in the form of optic neuritis, pyramidal-tract symptoms, brainstem, and spinal-cord symptoms.3,4 Typically 5–10% of patients have primary progressive multiple sclerosis (PPMS), with the most-common presentation of a spinal-cord syndrome without a sensory level.5 Secondary progressive multiple sclerosis (SPMS) is seen at 12–19 years after the RRMS onset, when there is gradual worsening of disability with or without occasional relapses and minor remissions.6,7,8 Recent advances in neuroimaging and biomarker research have resulted in SPMS being further defined for better understanding of the exact phenotype and its course.
The epidemiology of MS has been studied extensively and reported mainly for Western countries due to high prevalence of MS in Caucasians.9,10 The variation in prevalence is interrelated with geographical location and genetic factors. While the prevalence of MS is higher in Northern Europe, America, and South Australia than in Asia and Africa, there have been many reports of MS increasing in parts of the world with a low prevalence.11,12,13 The purpose of the present study was to determine the prevalence and clinical presentation of MS patients in the Saudi population in order to obtain a better understanding among physicians of the disease activity, prognosis, and decision-making. This will open a different horizon for future research and management decisions in these patients.
METHODS
This retrospective cross-sectional observational study was carried out in the Department of Neurology, King Fahd Hospital of University Alkhobar in the Kingdom of Saudi Arabia (KSA) from April to October 2017 after Institutional Review Board approval (IRB No. 2017-01-027) had been received from the university. Data were obtained for 224 male and female patients aged >14 years who presented to the hospital with a suspected demyelinating disorder and who were investigated with brain and spine MRI with gadolinium enhancement from January to December 2016. Some of the initially enrolled patients out to have MS-mimicking conditions such as neuromyelitis optica, acute disseminated encephalomyelitis, and vasculitis, and they were excluded from study. Only 190 patients who fulfilled the McDonald criteria for MS who had regular findings in subsequent follow-up MRI and patients with CIS were included in this study.
The different phenotypic patterns of disease were defined as follows:
CIS
The presence of a first clinical attack with objective evidence of neurological changes and a lesion compatible with MS in MRI studies.
RRMS
A history of at least two clinical attacks or at least one clinical attack with new documented MRI lesions over time. Relapse was defined as an acute deterioration of neurological function lasting for at least 24 hours followed by a period of total or partial recovery.
SPMS
Evolving in patients previously with a history of clinical attack (RRMS) who demonstrated gradual and progressive disability with or without periods of relapse.
PPMS
Gradual and progressive worsening of neurological function from the beginning of the disease with or without subsequent relapses observed at least 1 year.
The collected data were entered into and analyzed on the Statistical Package for Social Sciences (version 20.0, IBM Corp., Armonk, NY, USA). The results were computed as frequency and percentage values for age, sex, race, age at onset, disease duration, first clinical presentation, other established clinical symptoms, disease phenotype, Expanded Disability Status Scale (EDSS) score, and sites of MRI lesions, gadolinium-enhanced lesions, and treatment group. Mean±SD values for age, age at disease onset, and duration of disease were calculated. Subgroup analyses were performed for each disease phenotype. The chi-square test was used to compare categorical variables and a probability value of <0.05 was taken as indicative of statistical significance.
RESULTS
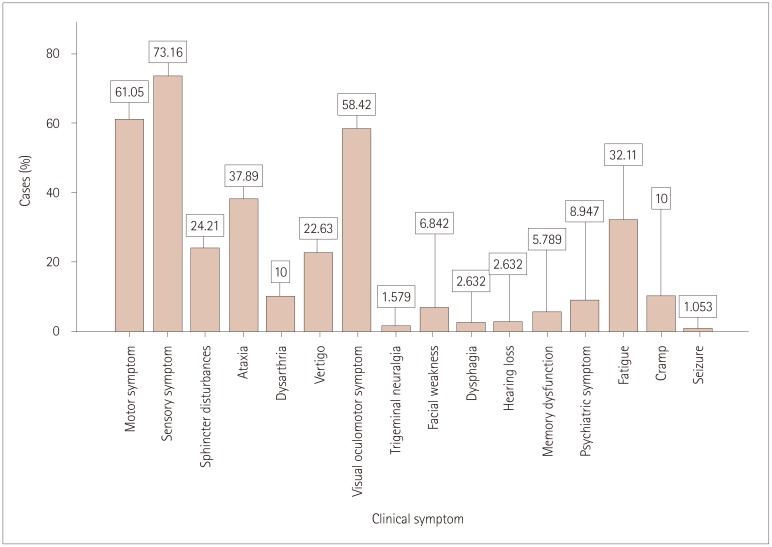
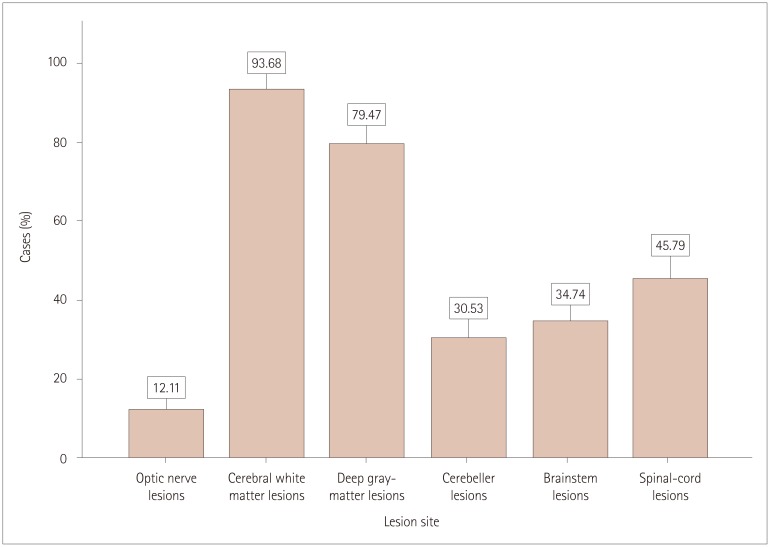
The demographic and clinical characteristics of the 190 patients with MS included in this study are presented in Table 1 and 2. About two-thirds of the participants were female (n=160, 62.6%), giving a male-to-female ratio of 1:1.6. The cases were aged 32.6±8.8 years, and their age at disease onset was 26.27±8.2 years, while the disease duration was 6.38±5.10 years. Most (n=179, 94.2%) of the cases were of Saudi origin. The 190 cases comprised 144 (75.8%) of RRMS, 23 (12.1%) of SPMS, 18 (9.5%) of CIS, and 5 (2.6%) of PPMS. Age, sex, disease duration, and the number of MRI gadolinium-enhanced lesions in each phenotype are presented in Table 3. Optic neuritis and myelitis were the most-frequent first clinical presentations of the disease, comprising 53 (27.9%) of cases, while brainstem and pyramidal-tract manifestations comprised 42 (22.1%) of the cases of first clinical attack. Other subsequent clinical manifestations are presented in Fig. 1. The frequency and distribution of clinical symptoms in each phenotype are further presented in Table 4. The frequencies of T2-weighted MRI lesions in the cases are presented in Fig. 2.
Table 1. Demographic characteristics of cases with multiple sclerosis (n=190).
| Gender, n (%) | |
| Male | 72 (37.9) |
| Female | 118 (62.1) |
| Ethnicity, n (%) | |
| Saudi | 179 (94.2) |
| Non-Saudi | 11 (5.8) |
| Age (years) | 32.6±8.8 (15–58) |
| Age at onset (years) | 26.2±8.2 (9–54) |
| Disease duration (years) | 6.38±5.10 (1–20) |
Data are n (%) or mean±SD (range) values.
Table 2. Clinical characteristics of cases with multiple sclerosis (n=190).
| Characteristic | n (%) |
|---|---|
| First clinical presentation/relapse | |
| Optic neuritis | 53 (27.9) |
| Myelitis | 53 (27.9) |
| Brainstem | 42 (22.1) |
| Pyramidal tract | 42 (22.1) |
| Disability score (EDSS) | |
| 1.0–5.5 | 156 (82.1) |
| 6.0–6.5 | 27 (14.2) |
| 7.0–7.5 | 7 (3.7) |
| 8.0–8.5 | 0 (0.0) |
| Distribution of lesions in MRI | |
| One system/site | 18 (9.5) |
| Two systems/sites | 47 (24.7) |
| Three systems/sites | 64 (33.7) |
| Four systems/sites | 44 (23.2) |
| Five systems/sites | 17 (8.9) |
| Topography of T2-weighted lesions in MRI | |
| Optic nerve | 23 (3.6) |
| Supra tentorial cerebral white matter | 178 (27.9) |
| Deep gray matter | 151 (23.7) |
| Cerebellum | 58 (9.1) |
| Brainstem | 66 (10.4) |
| Spinal cord | 87 (13.7) |
| DMT | |
| Patients not on any DMT treatment | 32 (16.8) |
| IFNβ-1a | 128 (67.4) |
| IFNβ-1b | 18 (9.5) |
| Other/oral | 12 (6.3) |
Data are n (%) values.
DMT: disease-modifying therapy, EDSS: Expanded Disability Status Scale, IFNβ: interferon β.
Table 3. Age, gender, age at onset, disease duration, and gadolinium-enhanced lesions in each multiple sclerosis phenotype (n=190).
| Clinical type | Total (%) | Females (%) | Males (%) | Age (years) | Onset age (years) | Disease duration (years) | Patients with gadolinium-enhanced lesion (%) |
|---|---|---|---|---|---|---|---|
| Clinically isolated syndrome | 18 (9.5) | 10 (55.6) | 8 (44.4) | 32.7±10.5 | 28.9±9.0 | 3.72±4.30 | 4 (22.1) |
| Relapsing-remitting multiple sclerosis | 114 (75.8) | 91 (63.2) | 53 (36.8) | 32.0±8.3 | 25.9±7.8 | 5.94±4.60 | 56 (38.9) |
| Secondary progressive multiple sclerosis | 21 (11.1) | 15 (71.4) | 6 (28.6) | 35.6±9.9 | 24.8±9.7 | 10.8±5.2 | 12 (57.1) |
| Primary progressive multiple sclerosis | 7 (3.7) | 2 (28.6) | 5 (71.4) | 39.7±7.4 | 31.0±7.9 | 8.7±7.1 | 2 (28.6) |
Data are n (%) or mean±SD values.
Fig. 1. Frequencies of different clinical symptoms (n=190).
Table 4. Frequency and distribution of clinical symptoms in each multiple sclerosis phenotype.
| Clinical symptom | Clinical subtype (%) | |||
|---|---|---|---|---|
| Clinically isolated syndrome 18 (9.5) | Relapsing-remitting multiple sclerosis 114 (75.8) | Secondary progressive multiple sclerosis 21 (11.1) | Primary progressive multiple sclerosis 7 (3.7) | |
| Motor | 6 (33.3) | 85 (59.3) | 21 (91.3) | 4 (66.7) |
| Sensory | 9 (50) | 104 (72.2) | 22 (95.7) | 6 (100) |
| Sphincter disturbances | 2 (11.1) | 30 (20.8) | 11 (47.8) | 2 (33.3) |
| Ataxia | 2 (11.1) | 52 (36.1) | 16 (69.6) | 3 (50) |
| Dysarthria | 2 (11.1) | 12 (8.3) | 4 (17.4) | 1 (16.7) |
| Vertigo | 1 (5.6) | 37 (25.7) | 5 (21.7) | 1 (16.7) |
| Visuo-ocular disturbances | 7 (38.9) | 89 (61.8) | 14 (60.9) | 2 (33.3) |
| Trigeminal neuralgia | - | 3 (2.1) | - | - |
| Facial weakness | 1 (5.6) | 8 (5.6) | 4 (17.4) | - |
| Dysphagia | 1 (5.6) | 3 (2.1) | 1 (4.3) | |
| Hearing loss | 3 (2.1) | 2 (8.7) | - | |
| Memory disturbances | - | 6 (4.2) | 5 (21.7) | 1 (16.7) |
| Psychiatric manifestations | 10 (6.6) | 6 (26.1) | 1 (16.7) | |
| Fatigue | - | 45 (31.3) | 14 (60.9) | 1 (16.7) |
| Cramps | - | 12 (8.3) | 5 (21.7) | 1 (16.7) |
| Seizures | 1 (5.6) | 1 (0.7) | - | - |
Data are n (%) values.
Fig. 2. Frequencies of T2-weighted MRI lesion sites (n=190).
In more than two-thirds of the cases (n=156, 82.1%) the EDSS scores were within the range of 1.0–5.5, while in 27 (14.2%) cases the EDSS scores were within the range of 6.0–6.5, and only 7 (3.7%) cases had EDSS scores within the range of 7.0–7.5. No case had an EDSS score of 7.5 in this study. More than half of the 128 (67.4%) patients were taking interferon β-1a (IFNβ-1a), and 18 (9.5%) were taking IFNβ-1b while only 12 (6.3%) were receiving a different oral disease-modifying therapy (DMT), 4 (2.1%) were taking natalizumab and 32 (16.8%) were not being treated.
DISCUSSION
The prevalence of MS is changing rapidly in different parts of the world.14,15,16 There are many factors underlying these changes, such as lifestyle modifications, changes in certain environmental factors, or simply an increased awareness and better diagnosis of the disease.17,18,19,20,21 Similar studies from the Middle East and other parts of the Arab world have shown variable prevalence rates of MS.22 The prevalence and pattern of clinical presentation have been studied previously in the KSA.23,24
The demographic data obtained this study are similar to those in other local studies, with a few notable differences. This study found that MS is more common in females than males, with a male-to-female ratio of 1:1.6. Daif et al.24 reported similar observations for a hospital-based study in KSA,24 while Yaqub reported that this ratio was 4.3 in another study from KSA.23 As with other autoimmune disorders, there is a slight female dominance worldwide, with a small amount of variability in the ratio in different parts of world, such as 3.0 in East Asia and 2.6 in the USA. There was a slightly higher number of male patients with MS in KSA that resulted in this ratio being different from that in the Yaqub study.23
The age at onset in our study was 26.27±8.2 years, which is almost same as that of 27.7±7.8 years in the study of Daif et al.24 The overall mean age at onset in the Middle East is 28.54 years, which is similar to that in Western countries and other parts of the world. The youngest age at presentation in our study was 9 years, for which the diagnosis was of pediatric MS, and this patient later presented in an adult neurology clinic at 14 years of age. The oldest age at presentation was 58 years. The youngest age at presentation in the literature is before 10 years, and MS is rarely seen in the eighth decade of life.25,26 The duration of disease in the present study was 6.38±5.11 years, ranging between 1 and 20 years, whereas other studies from the Middle East have found disease durations of 3.9±9.3 and 5.5±4.7 years.13,27
In this study optic neuritis and myelitis were the most-frequent first clinical presentations of disease, while brainstem and pyramidal-tract findings as well as ataxia, vertigo, and hemisensory loss were less-frequent first clinical presentations. These findings are consistent with other studies from the region.20,28,29 Optic-nerve and spinal-cord involvement, with predominant visual impairment in the beginning of the course, is more commonly reported in Asian countries.
Among other symptoms, fatigue was reported in one-third of our cases. A study from Pakistan found fatigue in 25% of cases.30 Dysarthria, psychiatric, and memory disturbances were also seen in up to 10% of the cases in our study. Other cranial-nerve symptoms and signs including facial weakness, hearing loss, and trigeminal neuralgia were seen in up to 6% of cases. Seizures were seen only in two patients (1%).
The clinical phenotype was RRMS in 75% of our cases and 60.7% in the study of Daif et al.24 A higher frequency is probably attributable to early diagnoses resulting from the better availability of services and imaging facilities along with modifications and revisions of diagnostic criteria for MS. An earlier diagnosis can lead to treatment starting earlier and reducing the probability of RRMS converting to SPMS over time. The frequency of SPMS was 11.1% in our study, and only 3.7% of the cases had PPMS; in contrast Daif et al.24 observed no cases of SPMS, while 19.1% of their cases were PPMS and 20% were progressive-relapsing multiple sclerosis (PRMS). These findings need to be reconsidered since PRMS has been eliminated and categorized as PPMS with activity and CIS is now considered part of the spectrum of MS after the 2013 revisions by the international advisory committee on clinical trials of MS.8 The clinical phenotype findings in our study were very similar to those found in recent studies from the United Arab Emirates and Kuwait.31,32
Supratentorial T2-weighted white-matter lesion lesions and deep-gray-matter or juxtacortical lesions were the most-frequent MRI lesions, comprising 28% and 23.7% of all MRI lesions observed in 93.6% and 79.4% of the cases, respectively. Optic-nerve and spinal-cord lesions were seen in 12.1% and 45.7% of the cases, respectively; despite their high presentation frequency clinically, cord lesions are small and peripheral and so they can be missed in conventional MRI. They can be detected more easily in short-time inversion recovery MRI sequences, and these are not obtained in all cases. Cerebellar and brainstem involvement were seen in 9–10% of the MRI lesions, which is similar to the rate seen in Western countries.33,34
Gadolinium-enhanced lesions comprised 11.62% of all lesions, and it was observed that their frequency was higher (57.1%) in SPMS cases than in RRMS (38.9%) and CIS (22.1%) cases. Two (28.6%) of the seven PPMS cases had gadolinium-enhanced lesions in this study, suggesting that a continuous inflammatory process was ongoing in cases of SPMS compared to other subtypes and that PPMS tends to be a more-progressive disease process that often presents with less-prominent gadolinium enhancement.35,36
About two-thirds of our patients (82.1%) had EDSS scores within the range of 1.0–5.5, reflecting functional mobility without requiring any walking aid. This indicates either a relatively benign course of disease or treatment-responsive disease in this population. However, studies from different parts of the world have also found mean EDSS scores of around 2 and 3. A Brazil study found that the EDSS score was 3.50±1.9,37 while a study of newly diagnosed MS from Qatar found a mean EDSS score of 2.4 (median=2), reflecting a lower level of clinical progression despite a higher radiological burden,38 and a study of 200 MS cases from Iran found that the EDSS score was 2.1±1.9.13 None of our patients had an EDSS score higher than 7.5 because patients with higher disability scores are bedridden, receives supportive treatment only.
Regarding the distribution of DMTs in this case series, 66.8% of the 127 patients were taking IFNβ-1a, including both subcutaneous and intramuscular on alternate days, and once-weekly regimens depending upon the physician decision and patient acceptance, tolerability, and drug availability. There were 18 (9.5%) patients taking IFNβ-1b, while only 12 (6.3%) of cases were receiving a different oral DMT at other centers, including teriflunomide (n=4), fingolimod (n=2) dimethylefumarate (n=2) and ocrelizumab (n=1) while 4 cases were started on natalizumab therapy due to treatment failure with IFNβ, with a more-severe and aggressive course of disease. Thirty-two (16.8%) patients were not receiving any treatment due to an advanced disease stage or patient refusal.
The present study had some limitations. Firstly, since this was a hospital-based retrospective cross-sectional study, the exact number of relapses and transitions of the disease spectrum from one clinical subtype to another could not be assessed. Secondly, we did not address other paraclinical parameters such as cerebrospinal fluid (CSF) analysis, oligoclonal bands, and evoked potentials, since these parameters have only appeared in new MS criteria.39 In particular, the presence of oligoclonal bands in the CSF with the first symptom is proposed as an alternative for waiting for another symptom or lesion for the early diagnosis of MS. Lastly, we could not determine the exact duration and any change in the treatment regimen according to a transition of disease spectrum due the cross-sectional nature of our study.
In conclusion, this study has shown that MS presenting in the hospital setting is more common in KSA then reported previously, and that the number of diagnosed cases is increasing. It is therefore an emerging and disabling neurological illness in KSA with clinical characteristics not dissimilar to those in other Middle Eastern countries. A decrease in the frequency of SPMS patients indicates that more new cases of RRMS are being diagnosed or that adequate treatments of RRMS are preventing the evolution to SPMS. Further larger and population-wide epidemiological and clinical studies with the long-term follow-up of MS patients are required to better assess the clinical spectrum of MS in KSA.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Richards RG, Sampson FC, Beard SM, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6:1–73. doi: 10.3310/hta6100. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 3.Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol. 1994;36(Suppl):S6–S11. doi: 10.1002/ana.410360704. [DOI] [PubMed] [Google Scholar]
- 4.Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012;11:157–169. doi: 10.1016/S1474-4422(11)70274-5. [DOI] [PubMed] [Google Scholar]
- 5.Rice CM, Cottrell D, Wilkins A, Scolding NJ. Primary progressive multiple sclerosis: progress and challenges. J Neurol Neurosurg Psychiatry. 2013;84:1100–1106. doi: 10.1136/jnnp-2012-304140. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler. 2003;9:260–274. doi: 10.1191/1352458503ms914oa. [DOI] [PubMed] [Google Scholar]
- 7.Scalfari A, Neuhaus A, Daumer M, Deluca GC, Muraro PA, Ebers GC. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 2013;70:214–222. doi: 10.1001/jamaneurol.2013.599. [DOI] [PubMed] [Google Scholar]
- 8.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans C, Beland SG, Kulaga S, Wolfson C, Kingwell E, Marriott J, et al. Incidence and prevalence of multiple sclerosis in the Americas: a systematic review. Neuroepidemiology. 2013;40:195–210. doi: 10.1159/000342779. [DOI] [PubMed] [Google Scholar]
- 10.Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13:128. doi: 10.1186/1471-2377-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alroughani R, Ahmed SF, Behbahani R, Khan R, Thussu A, Alexander KJ, et al. Increasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult Scler. 2014;20:543–547. doi: 10.1177/1352458513504328. [DOI] [PubMed] [Google Scholar]
- 12.Deleu D, Mir D, Al Tabouki A, Mesraoua R, Mesraoua B, Akhtar N, et al. Prevalence, demographics and clinical characteristics of multiple sclerosis in Qatar. Mult Scler. 2013;19:816–819. doi: 10.1177/1352458512459291. [DOI] [PubMed] [Google Scholar]
- 13.Kalanie H, Gharagozli K, Kalanie AR. Multiple sclerosis: report on 200 cases from Iran. Mult Scler. 2003;9:36–38. doi: 10.1191/1352458503ms887oa. [DOI] [PubMed] [Google Scholar]
- 14.Kingwell E, Zhu F, Marrie RA, Fisk JD, Wolfson C, Warren S, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991–2010) J Neurol. 2015;262:2352–2363. doi: 10.1007/s00415-015-7842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan S, Rose N, Masita A, Dhaliwal JS, Puvanarajah SD, Rafia MH, et al. Multiple sclerosis in Malaysia: demographics, clinical features, and neuroimaging characteristics. Mult Scler Int. 2013;2013:614716. doi: 10.1155/2013/614716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idris MN, Sokrab TE, Ibrahim EA, Ali HE, Elzibair MA, Abadalatif M, et al. Multiple sclerosis in Sudan: a prospective study of clinical presentation and outcome. Mult Scler. 2009;15:1537–1538. doi: 10.1177/1352458509345913. [DOI] [PubMed] [Google Scholar]
- 17.Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7:268–277. doi: 10.1016/S1474-4422(08)70042-5. [DOI] [PubMed] [Google Scholar]
- 18.Pugliatti M, Harbo HF, Holmøy T, Kampman MT, Myher KM, Riise T, et al. Environmental risk factors in multiple sclerosis. Acta Neurol Scand Suppl. 2008;188:34–40. doi: 10.1111/j.1600-0404.2008.01029.x. [DOI] [PubMed] [Google Scholar]
- 19.Naeem Z. Vitamin D deficiency- an ignored epidemic. Int J Health Sci (Qassim) 2010;4:V–VI. [PMC free article] [PubMed] [Google Scholar]
- 20.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–191. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohlega S, Inshasi J, Al Tahan AR, Madani AB, Qahtani H, Rieckmann P. Multiple sclerosis in the Arabian Gulf countries: a consensus statement. J Neurol. 2013;260:2959–2963. doi: 10.1007/s00415-013-6876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaqub BA, Daif AK. Multiple sclerosis in Saudi Arabia. Neurology. 1988;38:621–623. doi: 10.1212/wnl.38.4.621. [DOI] [PubMed] [Google Scholar]
- 24.Daif AK, Al-Rajeh S, Awada A, Al Bunyan M, Ogunniyi A, AbdulJabar M, et al. Pattern of presentation of multiple sclerosis in Saudi Arabia: analysis based on clinical and paraclinical features. Eur Neurol. 1998;39:182–186. doi: 10.1159/000007931. [DOI] [PubMed] [Google Scholar]
- 25.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–A394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 27.El-Salem K, Al-Shimmery E, Horany K, Al-Refai A, Al-Hayk K, Khader Y. Multiple sclerosis in Jordan: a clinical and epidemiological study. J Neurol. 2006;253:1210–1216. doi: 10.1007/s00415-006-0203-2. [DOI] [PubMed] [Google Scholar]
- 28.Etemadifar M, Abtahi SH, Akbari M, Murray RT, Ramagopalan SV, Fereidan-Esfahani M. Multiple sclerosis in Isfahan, Iran: an update. Mult Scler. 2014;20:1145–1147. doi: 10.1177/1352458513516531. [DOI] [PubMed] [Google Scholar]
- 29.Tallawy HN, Farghaly WM, Rageh TA, Shehata GA, Badry R, Metwally NA, et al. Door-to-door survey of major neurological disorders (project) in Al Quseir City, Red Sea Governorate, Egypt. Neuropsychiatr Dis Treat. 2013;9:767–771. doi: 10.2147/NDT.S36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahwar H, Akhter SW, Ali S. Clinical spectrum of multiple sclerosis at a tertiary care hospital in Pakistan. Pak J Med Sci. 2008;24:221–226. [Google Scholar]
- 31.Inshasi J, Thakre M. Prevalence of multiple sclerosis in Dubai, United Arab Emirates. Int J Neurosci. 2011;121:393–398. doi: 10.3109/00207454.2011.565893. [DOI] [PubMed] [Google Scholar]
- 32.Alroughani R, Ahmed SF, Al-Hashel J. Demographics and clinical characteristics of multiple sclerosis in Kuwait. Eur Neurol. 2014;72:181–185. doi: 10.1159/000362270. [DOI] [PubMed] [Google Scholar]
- 33.Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 34.Montalban X, Tintoré M, Swanton J, Barkhof F, Fazekas F, Filippi M, et al. MRI criteria for MS in patients with clinically isolated syndromes. Neurology. 2010;74:427–434. doi: 10.1212/WNL.0b013e3181cec45c. [DOI] [PubMed] [Google Scholar]
- 35.Ontaneda D, Fox RJ. Progressive multiple sclerosis. Curr Opin Neurol. 2015;28:237–243. doi: 10.1097/WCO.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka K, Kujuro Y, Suzuki S, Tanahashi N, Hamada J, Nogawa S, et al. Clinical and laboratory features of in-patients with multiple sclerosis in a University Hospital in Tokyo from 1988–2002. Intern Med. 2005;44:560–566. doi: 10.2169/internalmedicine.44.560. [DOI] [PubMed] [Google Scholar]
- 37.Negreiros AA, Sousa-Munõz RL, Oliveira BE, Nóbrega PV, Monteiro LL. Clinical and epidemiological profile of patients diagnosed with multiple sclerosis in João Pessoa, Paraiba, Brazil. Arq Neuropsiquiatr. 2015;73:741–745. doi: 10.1590/0004-282X20150111. [DOI] [PubMed] [Google Scholar]
- 38.Akhtar N, Elsetouhy A, Deleu D, Kamran S, AlHail H, Elalamy O, et al. Newly diagnosed multiple sclerosis in state of Qatar. Clin Neurol Neurosurg. 2013;115:1333–1337. doi: 10.1016/j.clineuro.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]