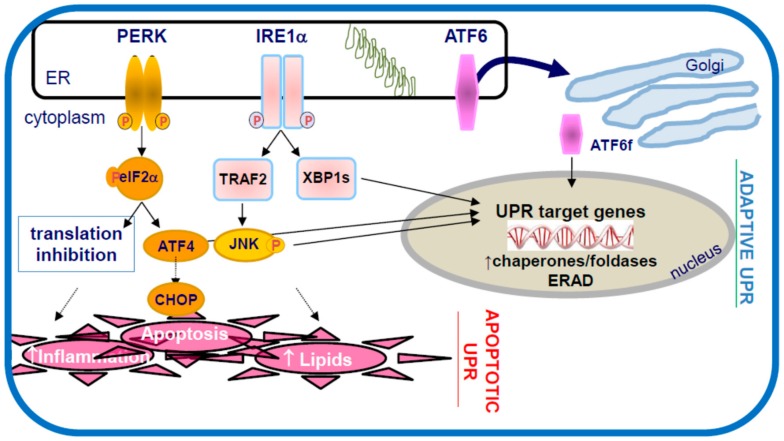
Figure 2.
The unfolded protein response to ER stress. The accumulation of misfolded proteins in the endoplasmic reticulum, defined as ER stress, activates three ER transmembrane signaling factors PERK, IRE1 and ATF6, which initiate the unfolded protein response (UPR). Initially the adaptive UPR acts to reestablish ER homeostasis by decreasing protein flux into the ER (translation block), increasing the folding capacity of the ER (increased chaperone expression) and enhancing the ER associated protein degradation (ERAD) pathways. Chronic ER stress results in the activation of C/EBP homologous protein (CHOP) and the pro-apoptotic UPR, which can include pro-inflammatory responses and lipid accumulation—hallmark features of atherosclerosis.