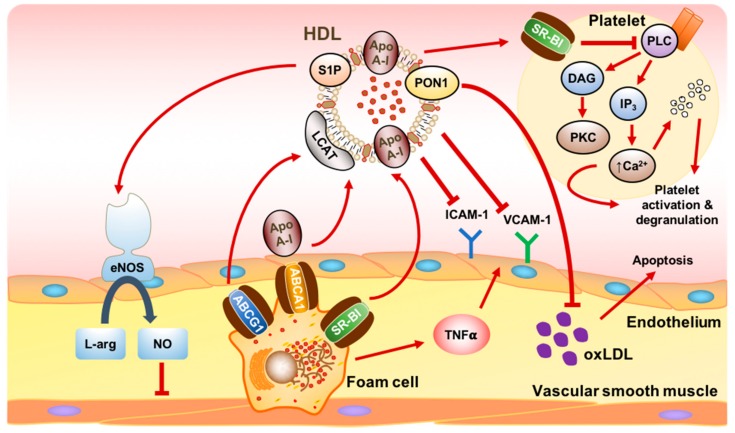
Figure 2.
Schematic representation of the diverse actions of HDL in atherosclerosis. HDL mediates reverse cholesterol transport by scavenging cholesterol from lipid-laden macrophages (foam cells) in atherosclerotic plaque. ApoA-I initiates cholesterol efflux by binding to ABCA1, with further uptake occurring via the ATP-binding cassette transporter G1 (ABCG1) and scavenger receptor class B type I (SR-BI). Cholesterol is esterified by lecithin-cholesterol acyl transferase (LCAT) and incorporated into the lipid core of mature HDL prior to transport to the liver for excretion. HDL also promotes endothelial function by stimulating endothelial nitric oxide synthase (eNOS) through the bioactive lipid shingosine-1-phosphate (S1P). eNOS converts l-arginine (l-arg) to nitric oxide (NO), which decreases vascular smooth muscle tone and reactivity. HDL exerts anti-apoptotic effects through paraoxonase-1 (PON-1), which hydrolyses oxidised LDL (oxLDL), a potent stimulator of apoptosis. HDL also inhibits the expression of recruitment factors for inflammatory cells, such as the intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1), which are stimulated in response to tumour necrosis factor α (TNFα) released by foam cells. Platelet activation is reduced by HDL through its interactions with SR-BI. HDL inhibits phospholipase C (PLC)-mediated production of diacylglycerol (DAG) and inositol trisphophate (IP3), which reduces protein kinase C (PKC) activation, intracellular calcium (Ca2+) mobilisation, and subsequent platelet degranulation. Red arrows denote a stimulatory effect while T bars denote an inhibitory effect.