Figure 4.
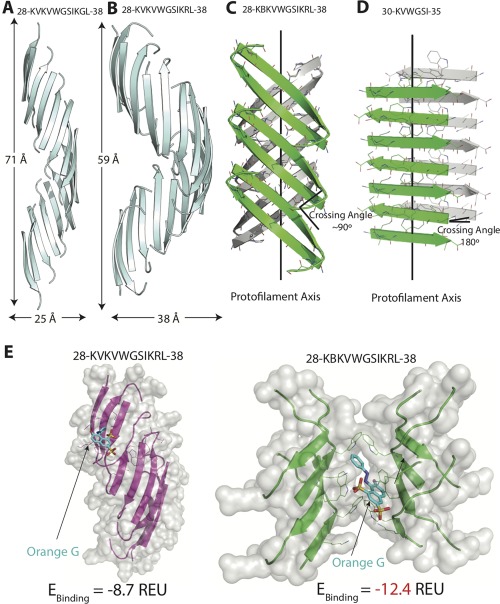
Structural comparison of SOD1 segment 28–38 and its variants. (A) The native 28–38 segment (PDB ID: 5DLI) forms a left‐handed corkscrew‐like assembly of antiparallel, out‐of‐register β‐strands. Sixteen strands form one complete turn with 71 Å pitch and 25 Å diameter. (B) The mutant segment with a familial mutation, G37R forms a corkscrew‐like assembly but with a smaller pitch and larger diameter. Twenty strands form one complete turn with 59 Å pitch and 38 Å diameter. (C) A second form of the variant segment reveals a novel architecture of curved out‐of‐register sheets. The β‐strands are not perpendicular to fibril axis and instead are tilted with a crossing angle of ∼90°. (D) A shorter (6‐residue) segment within 28–38 forms a steric zipper composed of pairs of β‐sheets. The β‐strands are perpendicular to the fibril axis with a 180° crossing angle. (E) Docking simulations of the different structures determined here and the small molecule dye orange G. Computed binding energy is reported in Rosetta Energy Units (REU). Notice that orange G has lower binding energy to curved β‐sheet than to corkscrew single sheet.
