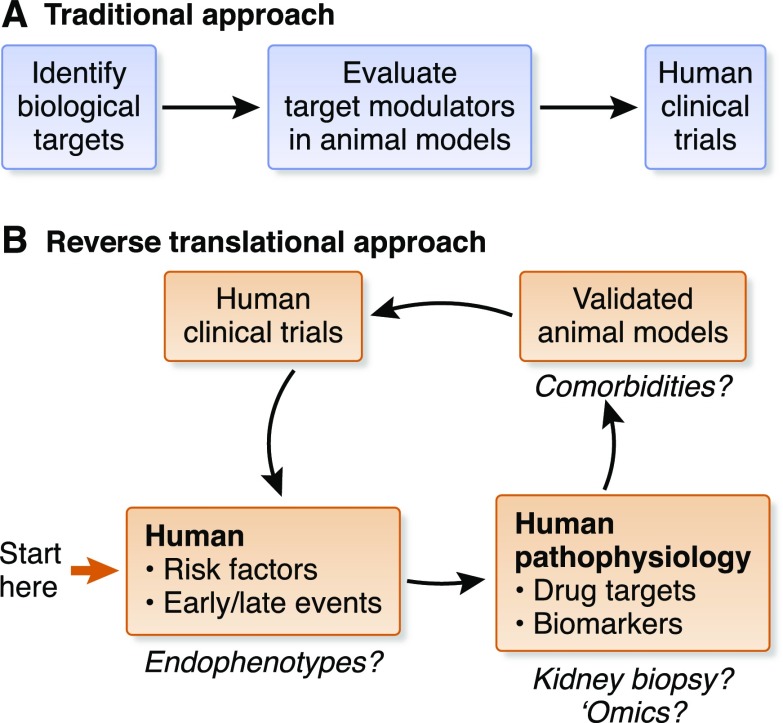
Figure 1.
Working backward iteratively to chart a path forward. In contrast to the traditional, linear approach to preclinical drug development (A), participants at the NIDDK “AKI Outcomes: Overcoming Barriers in AKI” workshop charted a path forward by starting with the ultimate goal: the necessary clinical trials (B). Participants considered first the specific patient population of interest, and then the resulting relevant human pathophysiology, including primary and secondary end points needed for such a study. Subsequently, participants identified the animal model that would replicate this patient population. By working backward, participants were able to identify considerable gaps in our knowledge and understanding of human AKI, and the relevance of AKI animal models and common biologic targets. Proposed gaps that were discussed by workshop participants are highlighted with question marks. Identification of endophenotypes may allow treatments to be tailored to patients who have the best chance of response to a therapeutic intervention. Kidney biopsy and advanced omics technologies for patient sample evaluations may allow for the identification of novel biologic targets and pathways, and provide important information on the fidelity of animal models, which can be fine-tuned to include comorbidities to more faithfully mimic the patient population.