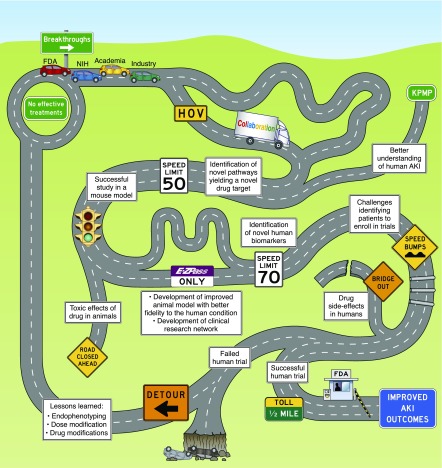
Figure 4.
Reaching improved AKI outcomes: the drug development journey. No effective treatments currently exist for AKI. A journey to achieve breakthroughs in AKI drug development requires involvement of academia, industry, the US Food and Drug Administration (FDA) and the National Institutes of Health (NIH), among others. Collaboration among these “travelers” may facilitate drug development. The Kidney Precision Medicine Project (KPMP) and other research efforts will improve our understanding of AKI, enabling identification of disease pathways and drug targets, thereby expanding the “roadway” of opportunities for drug development. Although identification of novel disease pathways, completion of successful animal studies, creation of improved animal models, and development of clinical research networks may enable more rapid advances, the process may be impeded by toxic effects of therapies in animals, challenges identifying patients to enroll in trials, or drug side effects, among other issues. Ultimately, reaching the destination of improved AKI outcomes will require execution of a successful human drug trial and approval of that therapy by the FDA. However, a failed human trial—even after much of the road has successfully been traversed—may send the travelers on a detour back to the start, with only some lessons learned to show for their journey. HOV, high occupancy vehicle.