Abstract
Objective
To investigate the pharmacokinetics, effectiveness, and safety of subcutaneous (SC) abatacept treatment over 24 months in patients with polyarticular‐course juvenile idiopathic arthritis (JIA).
Methods
In this phase III, open‐label, international, multicenter, single‐arm study, patients with polyarticular JIA (cohort 1, ages 6–17 years and cohort 2, ages 2–5 years) in whom treatment with ≥1 disease‐modifying antirheumatic drug was unsuccessful received weight‐tiered SC abatacept weekly: 10 to <25 kg (50 mg), 25 to <50 kg (87.5 mg), ≥50 kg (125 mg). Patients who had met the JIA–American College of Rheumatology 30% improvement criteria (achieved a JIA‐ACR 30 response) at month 4 were given the option to continue SC abatacept to month 24. The primary end point was the abatacept steady‐state serum trough concentration (Cminss) in cohort 1 at month 4. Other outcome measures included JIA‐ACR 30, 50, 70, 90, 100, and inactive disease status, the median Juvenile Arthritis Disease Activity Score in 71 joints using the C‐reactive protein level (JADAS‐71–CRP) over time, safety, and immunogenicity.
Results
The median abatacept Cminss at month 4 (primary end point) and at month 24 was above the target therapeutic exposure (10 μg/ml) in both cohorts. The percentage of patients who had achieved JIA‐ACR 30, 50, 70, 90, or 100 responses or had inactive disease responses at month 4 (intent‐to‐treat population) was 83.2%, 72.8%, 52.6%, 28.3%, 14.5%, and 30.1%, respectively, in cohort 1 (n = 173) and 89.1%, 84.8%, 73.9%, 58.7%, 41.3%, and 50.0%, respectively, in cohort 2 (n = 46); the responses were maintained to month 24. The median (interquartile range) JADAS‐71–CRP improved from baseline to month 4: cohort 1, from 21.0 (13.5, 30.3) to 4.6 (2.1, 9.4); cohort 2, from 18.1 (14.0, 23.1) to 2.1 (0.3, 4.4). Improvements were sustained to month 24, at which time 27 of 173 patients (cohort 1) and 11 of 22 patients (cohort 2) had achieved JADAS‐71–CRP remission. No unexpected adverse events were reported; 4 of 172 patients (2.3%) in cohort 1 and 4 of 46 (8.7%) in cohort 2 developed anti‐abatacept antibodies, with no clinical effects.
Conclusion
Weight‐stratified SC abatacept yielded target therapeutic exposures across age and weight groups, was well tolerated, and improved polyarticular JIA symptoms over 24 months.
Juvenile idiopathic arthritis (JIA) is a rheumatic disease presenting in children <16 years of age 1, 2, 3. For polyarticular‐course JIA (JIA of any category with ≥5 affected joints 1, 4, 5), methotrexate (MTX) is the recommended first‐line disease‐modifying antirheumatic drug (DMARD) therapy 3. If disease activity remains moderate or high after 3 months of MTX treatment, a biologic agent is frequently initiated and a tumor necrosis factor inhibitor (TNFi) is most commonly employed, followed by an anti–interleukin‐6 agent or abatacept 6. However, it is important to have multiple treatment options in this pediatric population, particularly for patients who are intolerant of or do not respond to MTX or TNFi 2, 7, 8, 9, 10, 11, 12, 13, 14, 15 or who live in a tuberculosis‐endemic country.
Abatacept, which selectively modulates the CD80/CD86:CD28 costimulatory signal required for full T cell activation, has a distinct mechanism of action upstream of that of other currently available treatments for rheumatic diseases 13, 16, 17, 18. In adults with rheumatoid arthritis (RA), intravenous (IV) abatacept inhibits structural damage, reduces disease progression, and improves function and health‐related quality of life, with good safety and tolerability 16, 19, 20. IV‐administered abatacept has been proven effective and well tolerated in polyarticular JIA clinical trials and real‐world settings 21, with benefits of treatment sustained up to 7 years in patients ages 6–17 years 2, 9, 13. A subcutaneous (SC) abatacept formulation is also available, allowing for administration at home, thus providing greater flexibility 22.
The exposure–response relationship established in RA and JIA has demonstrated that abatacept steady‐state serum trough concentration (Cminss) is a good predictor of efficacy 23, 24. A Cminss of 10 μg/ml has been shown to correlate with near‐maximal efficacy, based on response as assessed by the Disease Activity Score in 28 joints 24 in RA and the JIA–American College of Rheumatology 30% improvement criteria (JIA‐ACR 30) 21, 23, 25 in JIA. In adults with RA, a weight‐tiered 10 mg/kg monthly IV dose and 125 mg weekly SC fixed dose of abatacept have shown equivalent efficacy and comparable safety 22, but the effectiveness and safety of SC abatacept in polyarticular JIA have yet to be demonstrated in a clinical trial.
The aim of this study was to investigate the pharmacokinetics (PK), effectiveness, safety, and immunogenicity of weekly weight‐tiered treatment with SC abatacept in patients with active polyarticular JIA, including those ≥2 years of age, over a period of 24 months.
Patients and methods
Study design. This 24‐month, single‐arm, open‐label, international, multicenter, 2‐part, phase III study 26 was initiated in August 2013 and conducted across 48 centers worldwide by members of the Paediatric Rheumatology International Trials Organisation (PRINTO) 27 and the Pediatric Rheumatology Collaborative Study Group (PRCSG) (see Appendix A for investigators who participated in this study). The study included 2 age cohorts (cohort 1, ages 6–17 years and cohort 2, ages 2–5 years). During the first 4 months (part 1), patients received open‐label SC abatacept weekly, based on body‐weight tier at each study visit (10 to <25 kg [50 mg], 25 to <50 kg [87.5 mg], and ≥50 kg [125 mg]) (see Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract for rationale for the dosing regimen).
At month 4, JIA‐ACR 30 responders 28 were given the opportunity to participate in a 20‐month extension (part 2 of the study) and continue open‐label abatacept treatment according to the previous dosing regimen; part 1 and part 2 formed the 24‐month cumulative period. Also at month 4, JIA‐ACR 30 nonresponders were given the option to continue SC abatacept for an additional 3 months and to discontinue treatment if a JIA‐ACR 30 response was not achieved by month 7. After part 2, long‐term follow‐up began, in which patients who completed both parts of the study entered a post‐study drug access program. The study was conducted in accordance with the Declaration of Helsinki 29, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and local regulations. At each site, an institutional review board or independent ethics committee approved the protocol, consent forms, and any other written information provided to patients or their legal representatives.
Patients. Enrolled patients met the International League of Associations for Rheumatology criteria for JIA 4 in 1 of the following categories: extended oligoarticular JIA, polyarticular JIA rheumatoid factor (RF) positive, polyarticular JIA RF negative, enthesitis‐related arthritis, psoriatic arthritis, or systemic JIA (with systemic features absent for ≥6 months prior to enrollment). Patients also had a history of ≥5 joints with active disease and active articular disease at baseline, defined as ≥2 active joints and ≥2 joints with limitation of motion.
In addition, patients were naive to treatment with abatacept but may have had an inadequate response or prior intolerance to ≥1 nonbiologic or biologic DMARD, including TNFi. Patients with systemic JIA at onset were restricted to ≤10% of the study population, and those with a prior inadequate response to TNFi or other biologic DMARDs were restricted to ≤30% of the study population (see Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). All patients or their legal representatives provided written informed consent prior to study entry.
Study end points. The primary end point was abatacept Cminss in cohort 1 (ages 6–17 years) at month 4 of the study (end of part 1). Secondary end points included the proportion of patients in cohort 1 achieving JIA‐ACR 30 response by the end of part 1 and the proportion of patients with serious adverse events (SAEs), AEs, AEs leading to discontinuation, death, marked laboratory abnormalities, and positive immunogenic responses (defined as the presence of antibodies reactive with abatacept in serum) 30 in both cohorts over parts 1 and 2. Abatacept Cminss to month 24 (end of part 2) was an exploratory end point for both cohorts.
Prespecified exploratory efficacy end points for both cohorts included the proportions of patients achieving JIA‐ACR 30, 50, 70, 90, 100 responses or inactive disease (modified criteria), defined as absence of active joints, physician's global assessment of disease severity ≤10 mm, and C‐reactive protein (CRP) level ≤0.6 mg/dl (based on the normal range for CRP as defined by the central analysis site) 31 over time to the end of part 2. JIA‐ACR response rates were not corrected for MTX or corticosteroid use; however, if patients received an intraarticular corticosteroid injection, the treated joint was designated as active for the following 3 months. Other prespecified exploratory end points for cohort 1 included median post‐baseline values of the 6 JIA‐ACR core set variables: number of active joints, number of joints with limitation of motion, physician's global assessment, parent's global assessment of patient overall well‐being, cross‐culturally adapted and validated version of the Childhood Health Assessment Questionnaire disability index (C‐HAQ DI) 32, and laboratory marker of acute inflammation (CRP level). Post hoc analyses in both cohorts were conducted to determine the median Juvenile Arthritis Disease Activity Score in 71 joints using the CRP level (JADAS‐71–CRP) 33, 34 over time, and the proportion of patients achieving low disease activity according to the JADAS‐71–CRP (cutoff value in polyarthritis ≤3.8) and remission (defined as a JADAS‐71–CRP value of ≤1 for ≥6 months) 33, 35. In addition, post hoc subgroup analyses of JIA‐ACR response rates and proportions of patients achieving JADAS‐71–CRP low disease activity and remission in cohort 1 were performed using baseline concomitant MTX treatment, prior biologic treatment, and JIA category as subgroups (see Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract).
Analysis populations. In both cohorts, the PK analysis population for month 4 (end of part 1) comprised treated patients for whom PK measurements were collected in the 4–10‐day window after the most recent SC dose; these patients also had 7 consecutive weekly SC abatacept injections at the same dosage prior to month 4. The PK analysis population at the time points other than month 4 also comprised those patients with PK measurements collected in the 4–10‐day window after the most recent SC dose.
Effectiveness analyses were conducted on the intent‐to‐treat (ITT) population (defined as all treated patients) for both cohorts in part 1, as prespecified. For part 2, an “as‐observed” analysis was planned for both cohorts. However, in cohort 1 (ages 6–17 years), ITT analyses were also performed for the evaluation of effectiveness (sensitivity analysis), except for median post‐baseline values for JADAS‐71–CRP and the 6 JIA‐ACR core set variables over the cumulative period. In cohort 2 (ages 2–5 years), only as‐observed analyses were performed for part 2, as not all patients had completed treatment at the time of database locking.
Safety analyses were performed on the ITT population in both cohorts, taking into consideration all events during parts 1 and 2. Marked laboratory abnormalities criteria were prespecified (see Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). Immunogenicity was assessed in patients in the ITT population for whom ≥1 post‐baseline immunogenicity result was collected.
Statistical analysis, sample size, and power calculation. As the aim of this study was to assess the PK of SC abatacept treatment, it was not powered for statistical hypothesis testing. A sample size of ~160 patients for cohort 1 (ages 6–17 years) was planned to allow for assessment of PK, effectiveness, safety, and immunogenicity, resembling the sample size of the 4‐month lead‐in phase of the IV abatacept JIA study 13. Enrollment of ~160 patients into the 3 body‐weight tiers was predicted to ensure that the half‐width of the 90% confidence interval (90% CI) for Cminss would be within 18% of the true population mean for each body‐weight tier group, calculated based on a log‐transformed standard deviation for Cminss of 0.49. For JIA‐ACR 30 response, a sample of 160 patients would yield a precision of 7.4% for the half‐width of the 95% CI, assuming an underlying true responder rate of 64%, as seen with IV abatacept in JIA 13. For cohort 2 (ages 2–5 years), a sample size of ≥30 patients permitted initial descriptive assessment of PK, effectiveness, safety, and immunogenicity.
All effectiveness, safety, and PK analyses were descriptive, with no formal statistical testing. For effectiveness analyses using the ITT population, patients with missing data were imputed as nonresponders. CIs for proportions were computed using normal approximation, provided that the actual number of events was ≥5; otherwise, computed CIs were calculated using an exact method. In order to investigate the impact of immunogenicity on effectiveness, safety, and PK, a clinical review of the data on effectiveness, safety, and PK for patients who had positive immunogenic responses was performed.
Results
Patient disposition and baseline characteristics. A total of 219 patients entered part 1 of the study; 173 were ages 6–17 years (cohort 1) and 46 were ages 2–5 years (cohort 2). As shown in Figure 1, 132 patients (76.3%) in cohort 1 and 24 (52.2%) in cohort 2 completed both parts 1 and 2. In both cohorts, the discontinuation rate was low across both parts of the trial; overall, the main reasons for discontinuation were lack of efficacy, withdrawal of consent, and AEs. Treatment in the cumulative period was ongoing for 15 patients (32.6%) in cohort 2 at the time of database locking. Baseline demographic and disease characteristics for cohorts 1 and 2 are shown in Table 1. Whereas 41.0% of patients (71 of 173) in cohort 1 had a disease duration of ≥2 years, most patients (91.3% [42 of 46]) in cohort 2—which included patients as young as 2 years of age—had a disease duration of <2 years (median 0.5 years). At baseline, 78.6% of patients in cohort 1 and 80.4% in cohort 2 were receiving concomitant MTX. A protocol violation occurred in cohort 1, due to inclusion of 5 patients from noneligible JIA categories in the analysis. The median exposure to abatacept during the cumulative period was 24.3 months (range 1.9–28.0) in cohort 1 and 24.1 months (range 4.0–26.2) in cohort 2.
Figure 1.
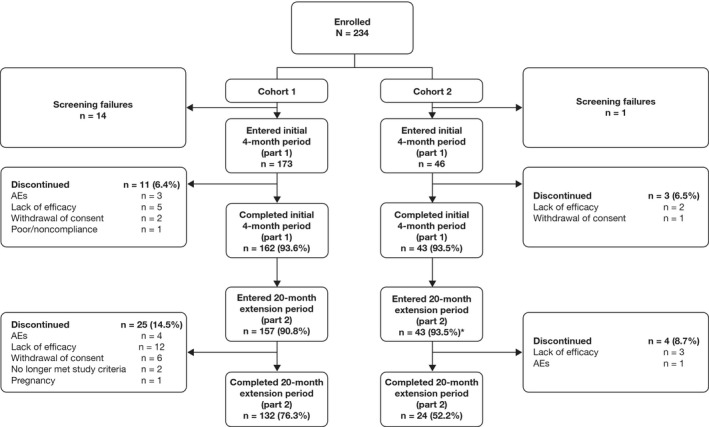
Patient disposition. * = treatment ongoing at time of analysis in 15 patients (32.6%). AEs = adverse events.
Table 1.
Summary of baseline demographics and clinical characteristics of the JIA patientsa
| Characteristic | Cohort 1 (n = 173) | Cohort 2 (n = 46) |
|---|---|---|
| Age, median (IQR) years | 13.0 (10.0–15.0) | 4.0 (3.0–5.0) |
| Female sex | 136 (78.6) | 28 (60.9) |
| Weight, median (IQR) kg | 45.0 (31.5–57.0) | 18.0 (15.0–21.1) |
| Weight category | ||
| <25 kg | 18 (10.4) | 43 (93.5) |
| 25 to <50 kg | 74 (42.8) | 3 (6.5) |
| ≥50 kg | 81 (46.8) | 0 |
| Ethnicity | ||
| White | 144 (83.2) | 44 (95.7) |
| African American | 14 (8.1) | 1 (2.2) |
| Other | 15 (8.7) | 1 (2.2) |
| Disease duration, median (IQR) | 2.0 (0.0–4.0) | 0.5 (0.0–1.0) |
| <2 years | 102 (59.0) | 42 (91.3) |
| 2 to <5 years | 37 (21.4) | 4 (8.7) |
| 5 to ≤10 years | 30 (17.3) | 0 |
| >10 years | 4 (2.3) | 0 |
| JIA category | ||
| Polyarthritis RF negative | 94 (54.3) | 29 (63.0) |
| Polyarthritis RF positive | 46 (26.6) | 3 (6.5) |
| Extended oligoarthritis | 19 (11.0) | 10 (21.7) |
| Systemic arthritis | 5 (2.9) | 0 |
| Psoriatic arthritis | 0 | 4 (8.7) |
| Enthesitis‐related arthritis | 4 (2.3) | 0 |
| Undifferentiated or persistent oligoarthritisb | 5 (2.9) | 0 |
| JIA‐ACR core set variable, median (IQR) | ||
| No. of active joints | 10.0 (6.0–19.0) | 7.0 (6.0–12.0) |
| No. of joints with limitation of motion | 8.0 (4.0–15.0) | 8.0 (4.0–11.0) |
| Physician global assessment | 48.0 (31.0–67.0) | 50.0 (35.0–60.0) |
| Parent's global assessmentc | 47.8 (24.1–68.0) | 42.1 (17.9–54.7) |
| C‐HAQ DIc | 0.9 (0.4–1.5) | 1.2 (0.8–1.6) |
| CRP, mg/dld | 0.2 (0.1–0.9) | 0.1 (0.1–1.4) |
| JADAS‐71–CRP, median (IQR)e | 21.0 (13.5–30.3) | 18.1 (14.0–23.1) |
| MTX use at baseline | 136 (78.6) | 37 (80.4) |
| MTX dose at baseline, median (IQR) mg/m2/week | 11.6 (9.7–14.4) | 13.3 (10.9–15.3) |
| Route of MTX administration | ||
| Oral | 76 (43.9) | 18 (39.1) |
| Parenteralf | 60 (34.7) | 19 (41.3) |
| Oral corticosteroid use at baselineg | 56 (32.4) | 9 (19.6) |
| Oral prednisone (or equivalent) dose at baseline, median (IQR) mg/kg/dayh | 0.1 (0.1–0.2) | 0.2 (0.2–0.4) |
| Prior biologic usei | 46 (26.6) | 10 (21.7) |
Except where indicated otherwise, values are the number (%) of patients. Cohort 1 consisted of patients ages 6–17 years, and cohort 2 consisted of patients ages 2–5 years. JIA = juvenile idiopathic arthritis; IQR = interquartile range; RF = rheumatoid factor; JIA‐ACR = JIA–American College of Rheumatology criteria for improvement; C‐HAQ DI = Childhood Health Assessment Questionnaire disability index; JADAS‐71‐CRP = Juvenile Arthritis Disease Activity Score in 71 joints using the C‐reactive protein level; MTX = methotrexate.
Protocol violation.
In cohort 1, n = 172.
Normal ≤0.6 mg/dl.
In cohort 1, n = 171.
Includes subcutaneous and intramuscular administration.
Prednisone or prednisolone.
In cohort 1, n = 52; in cohort 2, n = 8.
Adalimumab, etanercept, or tocilizumab.
Pharmacokinetics. The median abatacept Cminss values at months 4 and 24 in cohort 1 were 40.5 μg/ml (range 9.3–97.0; n = 135) and 43.0 μg/ml (range 0.0–105.2; n = 79), respectively, and were 51.2 (20.1–122.1; n = 30) and 58.7 (26.5–103.7; n = 19) in cohort 2, respectively (Figure 2). Across all weight groups in cohort 1 and overall in cohort 2, the median Cminss values were consistent and above the target therapeutic exposure of 10 μg/ml. After the effect of body weight was taken into account, there was no independent age effect on Cminss (see Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). The results of this study do not indicate that Cminss values >10 μg/ml were associated with either greater effectiveness or an increase in rates of AEs (including infections) at the month 4 time point (data not shown).
Figure 2.
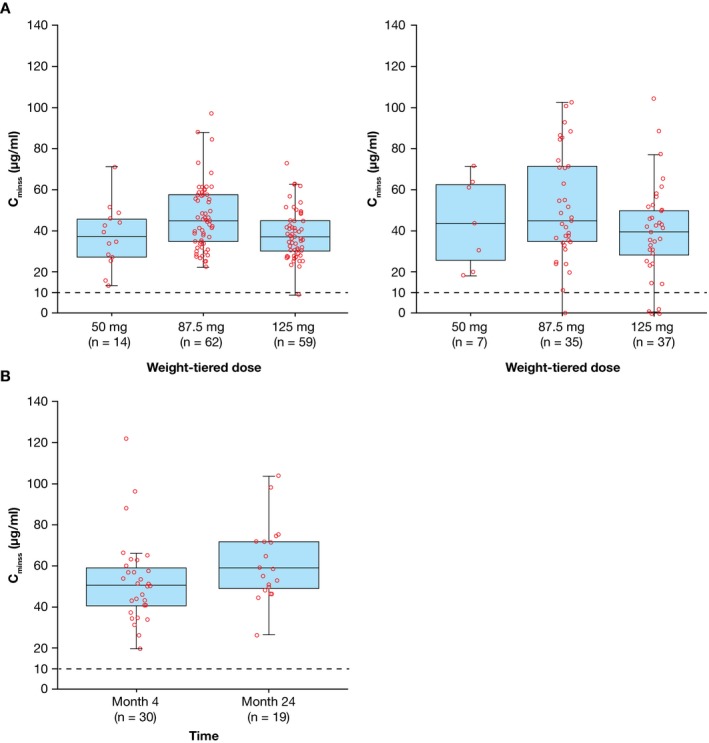
Abatacept steady‐state trough concentration (Cminss) in patients ages 6–17 years (cohort 1) by weight group (A) at month 4 (left) and month 24 (right), and patients ages 2–5 years (cohort 2) at months 4 and 24 (B). Cminss was summarized for treated patients (≥1 dose of study medication) with evaluable pharmacokinetic measurements at each time point. Data are shown as box plots. Each symbol represents a single subject. Boxes represent the upper and lower interquartile range. Lines inside the boxes represent the median. Whiskers represent the 5th and 95th percentiles. Dashed lines indicate the target therapeutic concentration.
Effectiveness. In both cohorts, robust JIA‐ACR responses were observed as early as month 1 (Figures 3A and B) (for “as observed” analysis, see Supplementary Materials). At month 4, JIA‐ACR 30 response rates were 83% and 89% in cohorts 1 (n = 173) and 2 (n = 46), respectively. JIA‐ACR 30 response rates in cohorts 1 and 2, respectively, were 75% and 96% at month 21, and 58% and 100% at month 24. Higher‐level JIA‐ACR response rates increased steadily to month 21 (see Figures 3A and B, and Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). Inactive disease status was achieved at month 4 by 30% and 50% of patients in cohorts 1 and 2, respectively. JIA‐ACR responses were in line with JADAS‐71–CRP scores, per the “as‐observed” analysis, reflecting low disease activity in 42.8% and 90.9% of patients in cohorts 1 and 2, respectively, at month 24. The median JADAS‐71–CRP values were below the cutoff value for low disease activity in polyarthritis by month 7 in cohort 1 and by month 3 in cohort 2; these values reached the lowest level of 1.3 at month 24 in cohort 1, and of 0.5 at month 19 in cohort 2 (Figures 4A and B). In cohort 1, all 6 JIA‐ACR core set variables, including C‐HAQ DI and parent's global assessment of patient overall well‐being, improved over time and these values were maintained until the end of the study (see Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). Additional results of JIA category analyses (cohort 1) can also be found in the Supplementary Materials.
Figure 3.
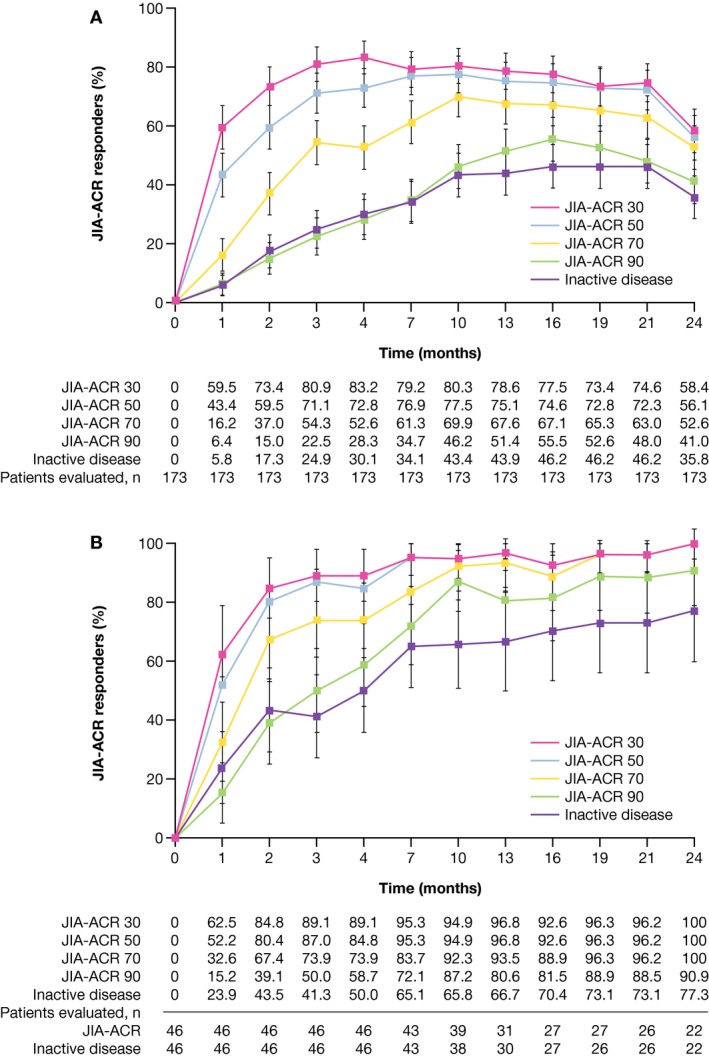
Juvenile Idiopathic Arthritis–American College of Rheumatology (JIA‐ACR) 30, 50, 70, 90 improvement criteria response rates over time for patients ages 6‐17 years (cohort 1; intent‐to‐treat [ITT] population analysis) (A) and patients ages 2‐5 years (cohort 2; ITT population analysis to month 4 [study part 1], “as‐observed” population analysis thereafter to month 24 [study part 2]) (B). Bars show the 95% confidence intervals. The apparent decrease in response rates at month 24 in cohort 1 reflects a decreased number of patients with available data at this time point and the associated increased proportion of patients imputed as nonresponders due to missing values.
Figure 4.
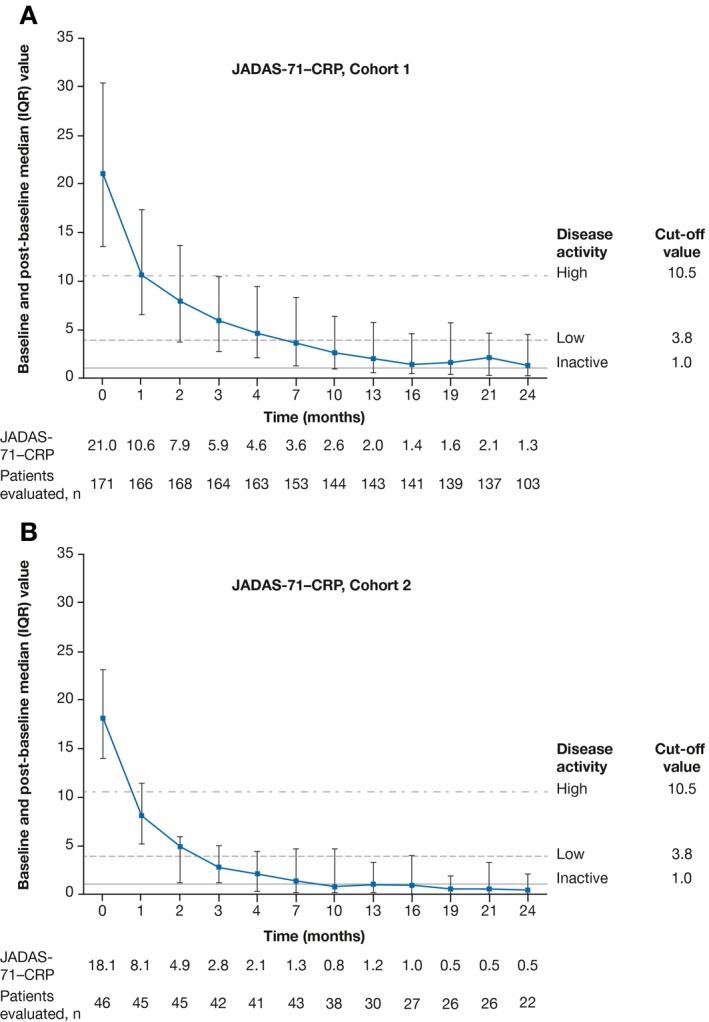
Baseline and post‐baseline Juvenile Arthritis Disease Activity Score in 71 joints using the C‐reactive protein level (JADAS‐71–CRP) in cohort 1 (A) and cohort 2 (B). Values are the median and interquartile range. JADAS‐71–CRP variables included number of active joints, physician's global assessment of disease activity, parent's global assessment of patient well‐being, and laboratory measurement of inflammation, as measured by CRP. Dashed lines show the JADAS‐71–CRP cutoff values for inactive disease, low disease activity, and high disease activity (1, 3.8, and 10.5, respectively) 33, 35.
Safety and immunogenicity. In total, 309.8 and 71.0 patient‐years of follow‐up were available from the safety populations in cohorts 1 and 2, respectively. As shown in Table 2, abatacept was well tolerated in both cohorts throughout the study. AEs occurred in 152 patients (87.9%) in cohort 1 and in 43 patients (93.5%) in cohort 2. SAEs occurred in 14 patients (8.1%) in cohort 1 and 3 (6.5%) in cohort 2. In cohort 1, the incidence rates of AEs and SAEs per 100 patient‐years of exposure were 173.0 (95% CI 147.6–202.8) and 4.7 (95% CI 2.8–7.9), respectively. Two SAEs were considered related to the study drug: sepsis (n = 1; severe intensity) in cohort 1 and overdose (n = 1; mild intensity) in cohort 2, in a patient in whom a higher abatacept dose was administered due to misclassification of weight tier. One malignancy, stage III ovarian germ cell teratoma (severe intensity) was reported in cohort 1 on study day 99, but was considered by the investigator to be unrelated to the study drug. Three autoimmune disorders (none of which were considered related to the study drug) were reported in cohort 1: pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (mild intensity) on study day 71, psoriasis (mild intensity) on study day 327, and Takayasu arteritis (moderate intensity) on study day 416. There were no reported cases of malignancies or autoimmune disorders in cohort 2.
Table 2.
Adverse events over the 24‐month cumulative period (all treated patients)a
| Event | Cohort 1 (n = 173) | Cohort 2 (n = 46) |
|---|---|---|
| Exposure, patient‐years | 309.8 | 71.0 |
| Deaths | 0 | 0 |
| SAEsb | 14 (8.1) | 3 (6.5) |
| Treatment‐related SAEsc | 1 (0.6) | 1 (2.2) |
| Discontinuations due to SAEs | 4 (2.3)d | 0 |
| Incidence rate, per 100 patient‐years | 4.68 | 4.41 |
| AEs (including SAEs) | 152 (87.9) | 43 (93.5) |
| Treatment‐related AEs | 54 (31.2) | 27 (58.7) |
| Discontinuations due to AEse | 7 (4.0) | 1 (2.2) |
| Incidence rate, per 100 patient‐years | 173.03 | 426.44 |
| AEs of special interestf | ||
| Malignancies | 1 (0.6) | 0 |
| Autoimmune disorders | 3 (1.7) | 0 |
| Local injection‐site reactions/pain | 12 (6.9) | 2 (4.3) |
| Infections | 118 (68.2) | 36 (78.3) |
Except where indicated otherwise, values are the number (%) of patients.
In cohort 1, serious adverse events (SAEs) included sepsis, abdominal pain, and upper respiratory tract infection (all 3 of which occurred in 1 patient), hypomagnesemia and stage III ovarian germ cell teratoma (both occurred in 1 patient), appendicitis, pneumonia, pyelonephritis, concussion, radius fracture, urinary calculus, nephrolithiasis, anemia, vertigo, chest pain, synovitis, and autonomic nervous system imbalance; in cohort 2, SAEs included overdose, tendon disorder, and febrile convulsion (each in 1 patient).
Treatment‐related SAEs included sepsis of severe intensity in cohort 1 and an overdose of mild intensity (administration of a higher abatacept dose due to misclassification by weight tier) in cohort 2.
Includes discontinuations due to the following SAEs: sepsis, vertigo, stage III ovarian germ cell teratoma, and autonomic nervous system imbalance.
Discontinuations due to sepsis, vertigo, stage III ovarian germ cell teratoma, autonomic nervous system imbalance, exanthema, fatigue, and aphthous ulcer in cohort 1; and pyrexia, rhinitis, and cough (all in 1 patient) in cohort 2.
No opportunistic infections (including extrapulmonary tuberculosis and herpes zoster) related to study drug occurred in either cohort during the study. In cohort 1, stage III ovarian germ cell teratoma was the only malignancy; autoimmune disorders included pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections, psoriasis, and Takayasu arteritis.
Local injection site reactions were of mild or moderate intensity and occurred in 12 patients (6.9%) in cohort 1 and 2 (4.3%) in cohort 2 (none of which led to discontinuation). Seven local injection site reactions of moderate intensity occurred in 3 patients in cohort 1, and 1 patient required treatment. The overall rates of patients with infections in cohorts 1 and 2, respectively, were 68.2% and 78.3%. All marked laboratory abnormalities were mild or moderate, and no deaths occurred during the study (for further details, see Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract).
Overall, 4 of 172 patients (2.3%) in cohort 1, and 4 of 46 (8.7%) in cohort 2 developed antibodies reactive with abatacept while receiving treatment during the cumulative period, but antibody positivity did not persist. The presence of these antibodies had no apparent effect on PK, effectiveness, or safety (see Supplementary Materials, available at http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract).
Discussion
In this phase III study of weekly weight‐tiered SC abatacept in patients with polyarticular JIA, the primary end point of abatacept Cminss at month 4 in patients ages 6–17 years was shown to be consistently above the planned minimal target therapeutic exposure of Cminss 10 μg/ml, without compromising patient safety. The target therapeutic exposure was achieved at month 4 across all weight groups in cohort 1, and overall in cohort 2. SC abatacept was effective and improved function and patient well‐being over the 24‐month cumulative treatment period in both cohorts, with a low rate of discontinuation due to AEs.
The PK data confirm the feasibility of a weekly weight‐tiered SC abatacept treatment regimen for polyarticular JIA in patients ages 2–17 years old (see Supplementary Materials, http://onlinelibrary.wiley.com/doi/10.1002/art.40466/abstract). The observed Cminss values were within the ranges of exposure previously found for IV abatacept (10 mg/kg) in patients with JIA 23 and in adults with RA receiving IV or SC abatacept treatment 36 (also see Supplementary Materials). Abatacept Cminss values at months 4 and 24 were comparable, demonstrating that the target therapeutic exposure is maintained with continued treatment.
The robust JIA‐ACR response rates and improvements in JADAS‐71–CRP scores to month 24 demonstrated in our study support the use of weekly weight‐tiered SC abatacept therapy in patients with polyarticular JIA. In both cohorts, an early onset of treatment response was observed, with responses maintained throughout the study. In cohort 1, analysis of the 6 JIA‐ACR core variables revealed a beneficial effect of SC abatacept on function (C‐HAQ DI) and well‐being (parent's global assessment of patient overall well‐being).
SC abatacept was well tolerated in both cohorts, with no new safety concerns observed. Safety and immunogenicity profiles were similar in cohorts 1 and 2. Although the rate of infections was higher than that observed in a study of adult patients with RA who were treated with abatacept 16, this finding was somewhat expected due to a known greater susceptibility of children to infections as compared with adults. Notably, there were no reported opportunistic infections related to abatacept treatment in patients with polyarticular JIA, even in countries with a high burden of tuberculosis, which is consistent with observations in adults with RA who receive abatacept treatment 37. In addition, there were no malignancies or autoimmune disorders in cohort 2, and the rate of these events was minimal in cohort 1. The safety of SC abatacept was largely consistent with that of IV abatacept in polyarticular JIA 2, 9, 13.
Tolerability of SC injections is of special concern in pediatric patients. In this study, SC abatacept treatment yielded an overall low rate of local injection site reactions, most of which were mild—only a few moderate and no severe reactions were reported. Notably, no local injection site reactions led to treatment discontinuation. Fewer patients developed anti‐abatacept antibodies while receiving SC abatacept treatment compared with IV abatacept treatment 2; this was somewhat expected given that similar trends were observed in a study of adult patients with RA who received SC or IV abatacept plus MTX and had inadequate responses to MTX 22. This finding was also possibly related to the concomitant use of MTX in ≥75% of patients in the current study and differences in study designs.
The effectiveness of SC abatacept treatment demonstrated in the current study constitutes a conservative interpretation of the data. Despite the observational character of the study, an ITT analysis was performed for the cumulative period in cohort 1 and up to month 4 in cohort 2, in which patients discontinuing the study prior to month 24 or not having data available at this time point were imputed as nonresponders.
Abatacept is seldom used to treat polyarticular JIA early in the disease course. The results from cohort 2 in this study, in which abatacept treatment was initiated in patients with a median disease duration of only 0.5 years, provide valuable data regarding the early introduction of abatacept therapy; notably, JIA‐ACR response rates were consistently higher in cohort 2 than in cohort 1.
The study has some limitations, including the open‐label design and a protocol violation, in which a few patients with undifferentiated and persistent oligoarthritis entered the trial despite not meeting the eligibility criteria. Additionally, the use of concomitant medications such as MTX and corticosteroids, as well as prior use of biologic DMARDs (including TNFi) in some patients, may have had confounding effects. However, the sample sizes of the corresponding subgroups were too small to analyze.
In conclusion, weight‐tiered weekly SC abatacept was effective in patients with polyarticular JIA, with no new safety concerns identified. These data suggest that SC abatacept provides an effective and well‐tolerated treatment option for patients with polyarticular JIA, with the additional benefit of the convenience associated with self or parent/caregiver administration of the treatment.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Dr. Brunner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Brunner, Lovell, Martini, Ruperto, Wong.
Acquisition of data
Brunner, Tzaribachev, Vega‐Cornejo, Louw, Berman, Calvo Penadés, Antón, Ávila‐Zapata, Cuttica, Horneff, Foeldvari, Keltsev, Kingsbury, Viola, Joos, Lauwerys, Paz Gastañaga, Rama, Wouters, Bohnsack, Breedt, Fischbach, Lutz, Minden, Miraval, Ally, Rubio‐Pérez, Solau Gervais, van Zyl, Lovell, Ruperto.
Analysis and interpretation of data
Brunner, Tzaribachev, Vega‐Cornejo, Louw, Berman, Calvo Penadés, Antón, Ávila‐Zapata, Cuttica, Horneff, Foeldvari, Keltsev, Kingsbury, Viola, Joos, Lauwerys, Paz Gastañaga, Rama, Wouters, Bohnsack, Breedt, Fischbach, Lutz, Minden, Miraval, Ally, Rubio‐Pérez, Solau Gervais, van Zyl, Li, Nys, Wong, Banerjee, Lovell, Martini, Ruperto.
Role of the study sponsor
The study was designed jointly by academic authors and Bristol‐Myers Squibb, with data collected by PRINTO/PRCSG investigators. The first and subsequent versions of the manuscript were written, edited, and revised critically by academic authors. Bristol‐Myers Squibb facilitated the study design and reviewed and approved the manuscript prior to submission. All authors independently collected the data, interpreted the results, had the final decision to submit the manuscript for publication, and had the right to accept or reject comments or suggestions. Writing assistance was funded by Bristol‐Myers Squibb. Publication of this article was not contingent upon approval by Bristol‐Myers Squibb.
Supporting information
Acknowledgments
The authors would like to thank Marco Garrone, PRINTO, for editorial assistance. Professional medical writing and editorial assistance was provided by Katerina Kumpan, PhD, at Caudex, and was funded by Bristol‐Myers Squibb. The authors are grateful to the protocol manager of this study, Mary Swingle, Bristol‐Myers Squibb.
Appendix A. Study investigators
The following investigators participated in the study: Mara L Becker (The Children's Mercy Hospital and Clinics, Kansas City, MO), Norman T Ilowite (Montefiore Medical Center, Bronx, NY), Jason A Dare (Arkansas Children's Hospital, Little Rock), Paula K Morris (Arkansas Children's Hospital, Little Rock), Timothy G Beukelman (The Children's Hospital of Alabama, Birmingham), Linda Wagner‐Weiner (The University of Chicago, Chicago, Illinois), Lawrence Zemel (Connecticut Children's Medical Center, Hartford), Pierre Quartier (Groupe Hospitalier Necker‐Enfants Malades, Paris, France), Isabelle Kone‐Paut (CHU de Bicêtre Service de pédiatrie, Kremlin‐Bicêtre Cedex, France), Alexandre Belot (Hôpital Femme Mère Enfant, Lyon, France), Valeria Gerloni (Istituto Ortopedico Gaetano Pini, Milan, Italy), Manuel Ferrandiz (Instituto Nacional de Salúd del Niño, Breña, Peru), Dina Janse Van Rensburg (Emmed Research, Eloffsdal, South Africa), Iloite Maria Scheibel (Hospital Nossa Senhora De Conceição, Porto Alegre, Brazil), Claudia Goldstein‐Schainberg (Hospital das Clínicas da Faculdade de Medicina da USP, São Paulo, Brazil), Clovis Silva (Hospital das Clínicas da Faculdade de Medicina da USP, São Paulo, Brazil), Maria Teresa Sande e Lemos Ascensao Terreri (UNIFESP‐Universidade Federal de São Paulo, São Paulo, Brazil), Maria Gamir (Hospital Universitario Ramón y Cajal, Madrid, Spain), Ruben Burgos Vargas (Hospital General de México, Mexico City, Mexico), Enrique Faugier Fuentes (Hospital Infantil de México Federico Gómez, Mexico City, Mexico), Rolando Cimaz (Ospedale Pediatrico Anna Meyer, Florence, Italy), Maria Alessio (Università degli Studi di Napoli Federico II, Naples, Italy) and Graciela Espada (Hospital de Niños Dr Ricardo Gutiérrez, Buenos Aires, Argentina).
ClinicalTrials.gov identifier: NCT01844518.
Supported by Bristol‐Myers Squibb.
Dr. Brunner has received consulting fees and/or speaking fees from AstraZeneca, Bristol‐Myers Squibb, Genentech, Janssen, Novartis, Pfizer, Sanofi, and Takeda (more than $10,000 each). Dr. Calvo Penadés has received speaking fees from AbbVie, Roche, Novartis, and Sobi (less than $10,000 each) and research support from Novartis. Dr. Antón has received speaking fees from AbbVie, Novartis, Pfizer, Roche, and Sobi (less than $10,000 each) and research support from Novartis and Pfizer. Dr. Horneff has received speaking fees from AbbVie, Pfizer, Chugai, Roche, and Novartis (less than $10,000 each). Dr. Lauwerys has received consulting fees from Bristol‐Myers Squibb, Servier, and Pfizer (less than $10,000 each) and research support from UCB, Janssen, and Pfizer. Dr. Paz Gastañaga has received research support from Pfizer, Novartis, Sanofi, and GlaxoSmithKline. Dr. Minden has received speaking fees and/or honoraria from AbbVie, Pfizer, Roche, and Pharm‐Allergan (more than $10,000 each) and research support from AbbVie, Pfizer, and Roche. Dr. Solau Gervais has received speaking fees from Bristol‐Myers Squibb, Pfizer, Roche, AbbVie, Novartis, and MSD (less than $10,000 each). Ms Li, Ms Nys, and Drs. Wong and Banerjee own stock or stock options in Bristol‐Myers Squibb. Dr. Lovell has received consulting fees and/or speaking fees from AbbVie, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Johnson & Johnson, Novartis, Takeda, and UCB (more than $10,000 each) and research support from AbbVie, Bristol‐Myers Squibb, Pfizer, and Roche. Dr. Ruperto has received consulting fees and/or speaking fees from AbbVie, Ablynx, Amgen, AstraZeneca, Baxalta Biosimilars, Biogen Idec, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eli Lilly and Company, EMD Serono, Gilead Sciences, Janssen, MedImmune, Novartis, Pfizer, R‐Pharm, Roche, Sanofi, Servier, and Takeda (more than $10,000 each).
Contributor Information
Hermine I. Brunner, Email: hermine.brunner@cchmc.org.
the Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG):
Mara L Becker, Norman T Ilowite, Jason A Dare, Paula K Morris, Timothy G Beukelman, Linda Wagner‐Weiner, Lawrence Zemel, Pierre Quartier, Isabelle Kone‐Paut, Alexandre Belot, Valeria Gerloni, Manuel Ferrandiz, Dina Janse Van Rensburg, Iloite Maria Scheibel, Claudia Goldstein‐Schainberg, Clovis Silva, Maria Teresa Sande e Lemos Ascensao Terreri, Maria Gamir, Ruben Burgos Vargas, Enrique Faugier Fuentes, Rolando Cimaz, Maria Alessio, and Graciela Espada
References
- 1. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 2. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Pérez N, Silva CA, et al. Long‐term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1792–802. [DOI] [PubMed] [Google Scholar]
- 3. Webb K, Wedderburn LR. Advances in the treatment of polyarticular juvenile idiopathic arthritis. Curr Opin Rheumatol 2015;27:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 5. Rosenberg AM, Oen KG. Polyarticular juvenile idiopathic arthritis In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, editors. Textbook of pediatric rheumatology. 7th ed Philadelphia: Elsevier Health Sciences; 2015. p. 217. [Google Scholar]
- 6. Beukelman T, Patkar NM, Saag KG, Tolleson‐Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular‐course juvenile idiopathic arthritis: results from a phase 3, randomised, double‐blind withdrawal trial. Ann Rheum Dis 2015;74:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunner HI, Ruperto N, Tzaribachev N, Horneff G, Chasnyk VG, Panaviene V, et al. Subcutaneous golimumab for children with active polyarticular‐course juvenile idiopathic arthritis: results of a multicentre, double‐blind, randomised‐withdrawal trial. Ann Rheum Dis 2018;77:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovell D, Ruperto N, Mouy R, Paz E, Rubio‐Pérez N, Silva CA, et al. Long‐term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol 2015;67:2759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al, for the Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 2000;342:763–9. [DOI] [PubMed] [Google Scholar]
- 11. Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810–20. [DOI] [PubMed] [Google Scholar]
- 12. Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo‐controlled trial of infliximab plus methotrexate for the treatment of polyarticular‐course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56:3096–106. [DOI] [PubMed] [Google Scholar]
- 13. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 14. Silverman E, Spiegel L, Hawkins D, Petty R, Goldsmith D, Schanberg L, et al. Long‐term open‐label preliminary study of the safety and efficacy of leflunomide in patients with polyarticular‐course juvenile rheumatoid arthritis. Arthritis Rheum 2005;52:554–62. [DOI] [PubMed] [Google Scholar]
- 15. Silverman E, Mouy R, Spiegel L, Jung LK, Saurenmann RK, Lahdenne P, et al. Leflunomide or methotrexate for juvenile rheumatoid arthritis. N Engl J Med 2005;352:1655–66. [DOI] [PubMed] [Google Scholar]
- 16. Genovese MC, Schiff M, Luggen M, Becker JC, Aranda R, Teng J, et al. Efficacy and safety of the selective co‐stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti‐tumour necrosis factor therapy. Ann Rheum Dis 2008;67:547–54. [DOI] [PubMed] [Google Scholar]
- 17. Orencia (abatacept) prescribing information. Princeton (NJ): Bristol‐Myers Squibb; 2017. URL: http://packageinserts.bms.com/pi/pi_orencia.pdf. [Google Scholar]
- 18. Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, et al. Efficacy and safety of abatacept, a T‐cell modulator, in a randomised, double‐blind, placebo‐controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Results of a two‐year follow up study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis Rheum 2008;58:953–63. [DOI] [PubMed] [Google Scholar]
- 20. Westhovens R, Kremer JM, Moreland LW, Emery P, Russell AS, Li T, et al. Safety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5‐year extended phase IIB study. J Rheumatol 2009;36:736–42. [DOI] [PubMed] [Google Scholar]
- 21. Lovell D, Ruperto N, Tzaribachev N, Zeft A, Cimaz R, Stanevica V, et al. Long‐term effectiveness and safety of abatacept in juvenile idiopathic arthritis: interim results from the abatacept in JIA registry [abstract]. Arthritis Rheumatol 2016;68 Suppl 10 URL: http://acrabstracts.org/abstract/long-term-effectiveness-and-safety-of-abatacept-in-juvenile-idiopathic-arthritis-interim-results-from-the-abatacept-in-jia-registry-2/. [Google Scholar]
- 22. Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 2011;63:2854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Passarell JA, Lin K, Roy A, Murthy B, Girgis IG. Population pharmacokinetics and exposure‐response analyses for abatacept in juvenile idiopathic arthritis [poster]. Presented at the American Conference on Pharmacometrics (ACoP8); 2017 October 15–18; Fort Lauderdale, FL. [Google Scholar]
- 24. Zhou Z, Roy A, Murthy B, Gao L, Teng J, Kaul S, et al. Relationship between systemic exposure and the efficacy and safety of abatacept administered subcutaneously and intravenously in adult rheumatoid arthritis patients [poster]. Presented at the American Association of Pharmaceutical Scientists National Biotechnology Conference; 2011 May 16–18; San Francisco, CA. [Google Scholar]
- 25. Ruperto N, Lovell DJ, Tzaribachez N, Vega‐Cornejo G, Louw I, Berman A, et al. Subcutaneous abatacept in patients with polyarticular juvenile idiopathic arthritis and inadequate response to biologic or non‐biologic disease‐modifying antirheumatic drugs: pharmacokinetics, efficacy and safety. Ann Rheum Dis 2016;75:138. [Google Scholar]
- 26. Bristol‐Myers Squibb, sponsor . A phase 3 multi‐center, open‐label study to evaluate pharmacokinetics, efficacy and safety of abatacept administered subcutaneously (SC) in children and adolescents with active polyarticular juvenile idiopathic arthritis (pJIA) and inadequate response (IR) to biologic or non biologic disease modifying anti‐rheumatic drugs (DMARDs). ClinicalTrials.gov identifier: NCT01844518; 2013.
- 27. Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 28. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 29. World Medical Association Declaration of Helsinki (1964) . Recommendations guiding physicians in biomedical research involving human subjects. BMJ 1996;313:1448. [Google Scholar]
- 30. Haggerty HG, Abbott MA, Reilly TP, DeVona DA, Gleason CR, Tay L, et al. Evaluation of immunogenicity of the T cell costimulation modulator abatacept in patients treated for rheumatoid arthritis. J Rheumatol 2007;34:2365–73. [PubMed] [Google Scholar]
- 31. Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, for the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the Pediatric Rheumatology Collaborative Study Group (PRCSG), and the Paediatric Rheumatology International Trials Organisation (PRINTO) . American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 32. Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado‐West L, et al. Cross‐cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries: review of the general methodology. Clin Exp Rheumatol 2001;19:S1–S9. [PubMed] [Google Scholar]
- 33. Nordal EB, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population‐based setting. Ann Rheum Dis 2012;71:1122–7. [DOI] [PubMed] [Google Scholar]
- 34. Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni‐Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 35. Consolaro A, Bracciolini G, Ruperto N, Pistorio A, Magni‐Manzoni S, Malattia C, et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum 2012;64:2366–74. [DOI] [PubMed] [Google Scholar]
- 36. Iwahashi M, Inoue H, Matsubara T, Tanaka T, Amano K, Kanamono T, et al. Efficacy, safety, pharmacokinetics, and immunogenicity of abatacept administered subcutaneously or intravenously in Japanese patients with rheumatoid arthritis and inadequate response to methotrexate: a Phase II/III, randomized study. Mod Rheumatol 2014;24:885–91. [DOI] [PubMed] [Google Scholar]
- 37. Louw I, Ally M, Janse van Rensburg DC, van Duuren E, Nel D, Miller‐Janson H, et al. Retention of use and safety of subcutaneous abatacept in rheumatoid arthritis: a patient record assessment in a compassionate use programme in South Africa, a tuberculosis endemic country [abstract]. Arthritis Rheumatol 2016;68 Suppl 10 URL: http://acrabstracts.org/abstract/retention-of-use-and-safety-of-subcutaneous-abatacept-in-rheumatoid-arthritis-a-patient-record-assessment-in-a-compassionate-use-programme-in-south-africa-a-tuberculosis-endemic-country/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


