Scheme 1.
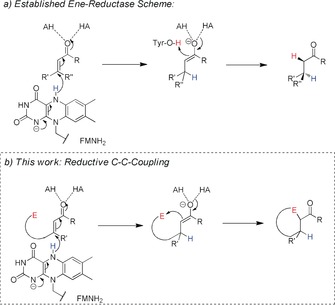
a) Reduction of an activated C=C bond by ene reductases. Activation of the double bond by hydrogen‐bond formation is enabled, for example, by two His residues (shown as “AH”/“HA”). The FMN hydride (shown in blue) is transferred to the β‐C. The resulting enolate is stabilized by two hydrogen bonds. Reprotonation at the α‐carbon occurs through a conserved tyrosine residue. b) Proposed mechanism of reductive C−C coupling. In the absence of the Tyr residue, the enolate attacks the internal electrophilic carbon, thereby enabling the formation of cyclic compounds.
