Figure 3.
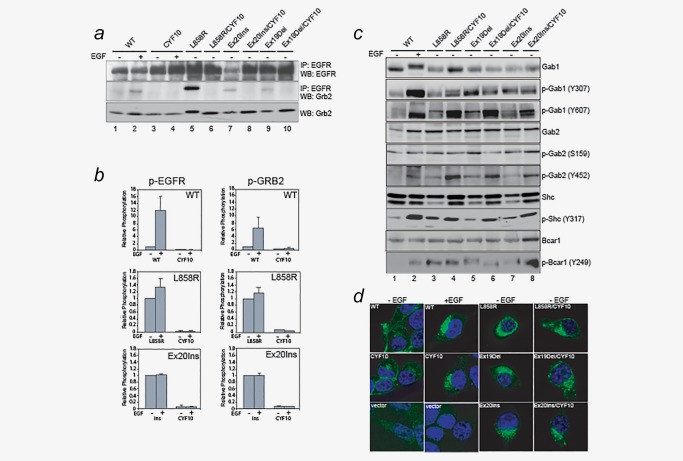
Shc1 and Bcar1, but not Grb2, may play a crucial role in induction of oncogenic signal activation by CYF10 mutants. (a) Grb2 adaptor proteins fail to associate with CYF10 mutants. Cell lysates prepared from NIH‐3T3 cells expressing L858R, Ex19Del, EX20Ins mutants or wild‐type EGFR with or without the CYF10 mutation were subjected to immunoprecipitation with anti‐EGFR antibody followed by immunoblotting for Grb2. The same blot was stripped and reprobed with anti‐EGFR antibody. The level of Grb2 expression was similar in all samples. (b) Phosphorylation of EGFR and Grb2 is abolished in CYF10 mutants. The levels of phospho‐EGFR and phospho‐Grb2 in CYF10 mutants were quantified by Luminex assays (see Experimental Procedures) in the presence or absence of EGF. The bar graph shows the relative levels of phosphorylation of EGFR and Grb2 in each CYF10 mutant normalized with to the respective EGF‐stimulated wild‐type EGFR, L858R or Ex20Ins mutants (n = 3, mean + SD). (c) Gab1/2, Shc1 and Bcar1 adaptor proteins were constitutively phosphorylated by mutant EGFR irrespective of C‐terminal phosphorylation. Cell lysates used in (a) were subjected to immunoblotting with antibodies against p‐Gab1/2, Gab1/2, p‐Shc1, Shc1 and p‐Bcar1. (d) The pattern of subcellular localization of kinase domain mutant EGFR was similar to that of EGF‐stimulated wild‐type EGFR, which is not affected by receptor C‐terminal phosphorylation. Confocal microscope images were acquired from the NIH‐3T3 stable cells described above after being fixed, permeablized and stained with FITC‐conjugated anti‐EGFR antibody.
