Figure 2.
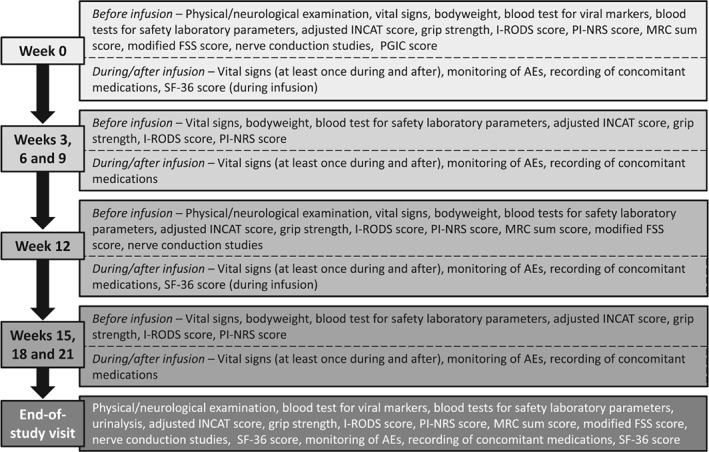
Schedule of study visit assessments during the dose‐evaluation phase. AEs, adverse events; FSS, fatigue severity scale; INCAT, inflammatory neuropathy cause and treatment; I‐RODS, inflammatory Rasch‐built overall disability scale; MRC, Medical Research Council; PGIC, Patients' global impression of change; PI‐NRS, pain intensity numeric rating scale; SF‐36, short form 36 items health status
