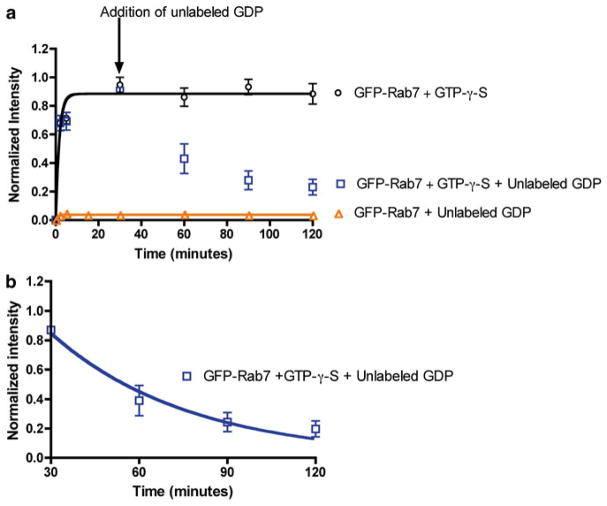
Fig. 3.
Quantitative measurements of Rab7 GTPase-RILP effector binding is rapid and nucleotide specific. (a) Flow cytometry-based measurement of the long-term kinetics of Rab7 binding to RILP by detecting fluorescent GFP-Rab7 shows binding is rapid and dependent on Rab7 being GTP bound. GFP-Rab7 prebound to nonhydrolyzable GTP-γ-S is nearly instantaneous and stable over 120 min. Addition of GDP results in displacement of GTP-γ-S from Rab7 and dissociation of GFP-Rab7GDP complex detected as a loss of bead-associated fluorescence. There is no binding of GFP-Rab7 in the GDP-bound state to RILP. (b) Data from panel (a) were replotted starting at the 30 min time point to allow determination of the dissociation rate of GFP-Rab7-GDP from RILP. Data was fitted to single-phase exponential decay function using PRISM software yielding a dissociation rate of 0.020 ± 0.003 min−1