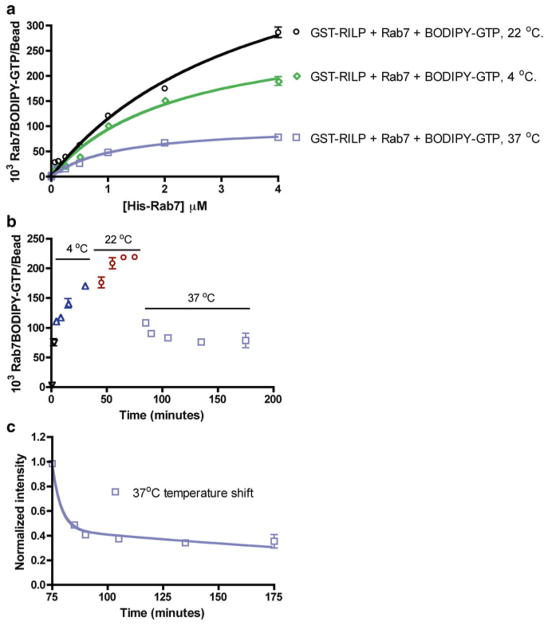
Fig. 4.
Flow cytometry-based measurements show Rab7 GTPase-RILP effector binding is temperature dependent and sensitive to nucleotide hydrolysis. (a–c) Flow cytometry-based measurement of His-Rab7 binding to RILP by detecting fluorescent BODIPY-GTP. (a) Dose-dependent His-Rab7 binding is temperature dependent and negatively affected by GTP hydrolysis at higher temperature (b and c). (b) A kinetic temperature-shift experiment shows His-Rab7 binding to RILP increases steadily at 4 and 22 °C, but decreases rapidly upon shift to 37 °C, likely due to GTP hydrolysis and dissociation of Rab7 from RILP. (c) Data in (b) were replotted starting at the 75 min time point to allow determination of the dissociation rate. Data were fitted to a two-phase exponential decay function using PRISM software yielding a dissociation rate of 0.014 ± 0.003 min−1 for the slow phase and 0.0436 ± 0.053 min-1 for the fast phase. The rate constant value deduced for the fast phase is statistically close to that measured for the dissociation of GFP-Rab7-GDP from RILP in Fig. 2a, supporting the conclusion that 37 °C stimulates Rab7 GTPase hydrolysis of BODIPY-GTP and consequent dissociation from RILP