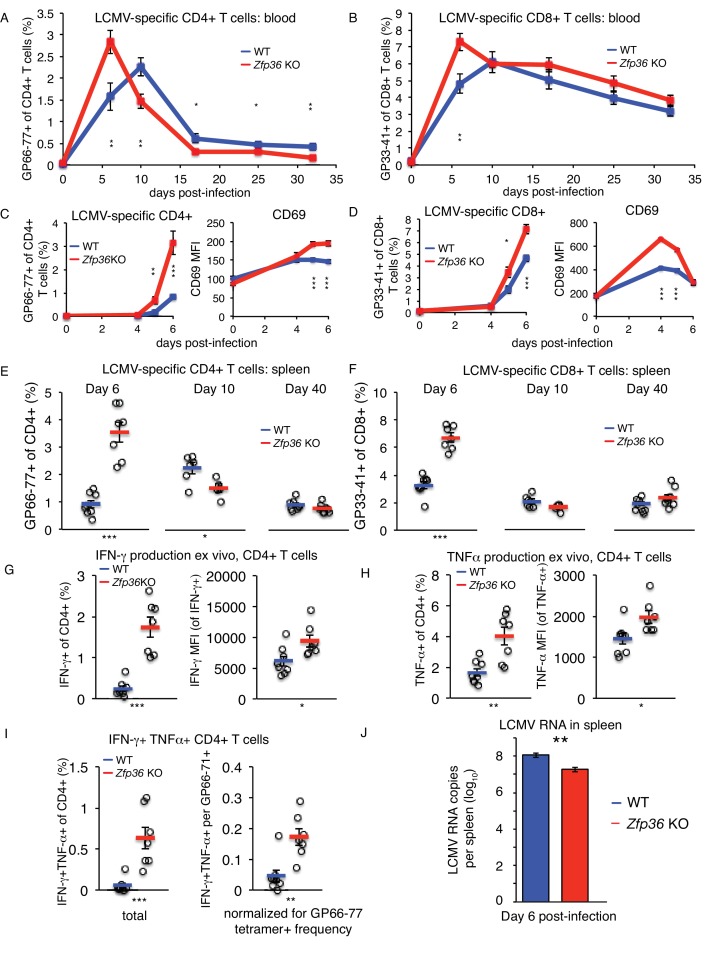
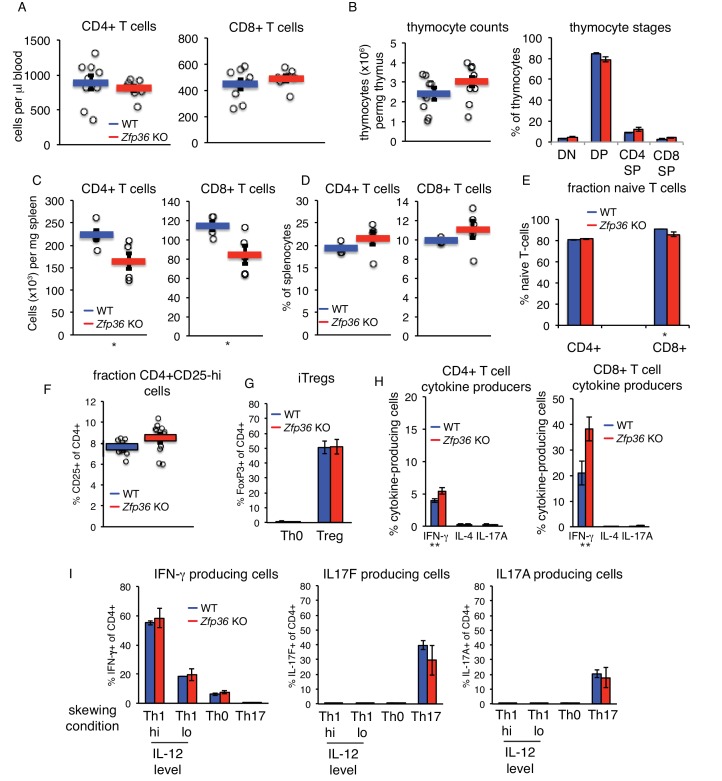
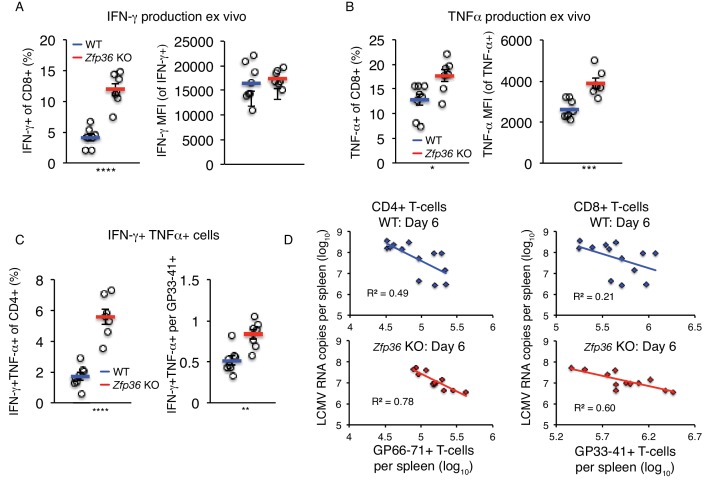
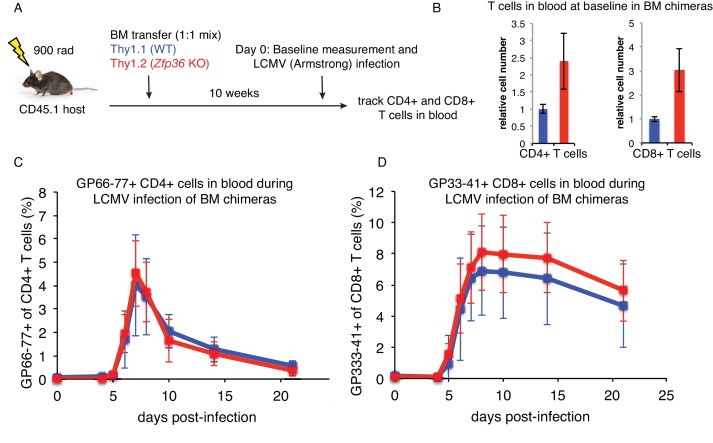
Figure 7. ZFP36 regulates anti-viral immunity.
(A) Virus-specific CD4 + or (B) CD8 +T cells were tracked in peripheral blood using MHC-tetramers after LCMV Armstrong infection (n = 8–9 mice per genotype). (C) Virus-specific CD4 +T cells and CD69 expression on CD4 +T cells in peripheral blood at early time points post-infection (p.i.) (n = 7–8 mice per genotype). (D) Virus-specific CD8 +T cells and CD69 expression on CD8 +T cells in peripheral blood at early time points p.i. (n = 7–8 mice per genotype). (E) Virus-specific CD4 +and (F) CD8 +T cells in spleen after LCMV infection (n = 5–8 mice per genotype). (G) Fraction of CD4 +T cells producing IFN-γ and TNF-α in splenic CD4 +T cells 6 days p.i., after ex vivo stimulation with GP66-77 peptide (n = 7–8 mice per genotype). (H) Levels of IFN-γ and TNF-α(gated on cytokine-producing CD4 +cells) 6 days p.i. after ex vivo stimulation with GP66-77 (n = 7–8 mice per genotype). (I) Raw percentage of bifunctional IFN-γ+TNF-α+CD4+cells in spleen 6 days p.i. after ex vivo stimulation with GP66-77 (left), or normalized to percentage of GP66-77 tetramer +cells (n = 7–8 mice per genotype). (J) Levels of LCMV genomic RNA in spleen measured by RT-qPCR (n = 9–14 per group). For (A–J), mean values ± S.E.M. are shown, with circles as individual mice. Results of two-tailed t-tests: *=p < 0.05; **=p < 0.01; ***=p < 0.001; ****=p < 0.0001. In each panel, one representative experiment of two is shown.