Abstract
Volatile oil in Chrysanthemum morifolium Ramat (C. morifolium) was extracted by the method of water vapor distillation and its chemical components was identified by gas-chromatography coupled with mass spectrometry (GC–MS). The volatile oil are evaluated for antibacterial activity against Escherichia coli, Staphylococcus aureus, Salmonella enteritids, Pseudomonas aeruginosa and Bacillus subtilis. Effects of surfactant, temperature, pH and ultraviolet light on antibacterial activity stability of volatile oil were analyzed too. Total 56 compounds were identified in C. morifolium volatile oil. The main constituents in C. morifolium volatile oil were monoterpenes and sesquiterpenes compounds, including hydrocarbons, esters, aldehydes, ketones, phenols and organic acids. α-curcumene was the most abundant volatile component (12.55%). The volatile oil showed promising antibacterial activity against 5 selected strains. The inhibitory effect on P. aeruginosa exhibited maximum inhibition zone diameter 20.43 mm, and E. coli showed 12.29 mm. The volatile oil treated with surfactant Tween 20 showed the strongest antibacterial activity, followed by Tween 80 and the SDS lowest, which showed the lowest. pH also had different effect on antibacterial activity stability of the C. morifolium volatile oil. No significant difference effect on antibacterial activity stability of volatile oil was observed with temperature and UV treatment.
Keywords: Chrysanthemum morifolium Ramat, Volatile oil, Chemical components, Antibacterial activity, Stability
Introduction
Chrysanthemum morifolium Ramat which belongs to the tribe Anthemideae in the Asteraceae family has been widely cultivated for more than 3000 years in China. The dry capitulum of the genera Chrysanthemum plants showed many benefits to human health. Its flowers are frequently taken in the manner of tea drinking, as well as used in Chinese traditional medicine (Matsuda et al. 2002). Furthermore, it’s one of the specialties in Zhejiang Province and one of the first batches of medicines approved by Chinese Health Ministry as authentic medicinal and edible plant (Wu et al. 2009).
Volatile oil is the odorous, volatile products of the secondary metabolism of plants by water steam distillation. They are used as fragrances and flavors in the perfume and food industries and recently as well as aromatherapy (Enan 2001; Isman 2006). Many reports had focused on volatile flavor composition and pharmacological effects of Chrysanthemum species (Choi and Kim 2011; Haouas et al. 2012; Chang and Kim 2013). Haouas et al. (2012) analyzed the components of three species of Chrysanthemum growing in Tunisia (C. coronarium, C. fuscatum, and C. grandiflorum) by GC–MS method. The result showed that the volatile oils obtained from leaves and flowers shared a similar qualitative composition, but the relative proportions of the constituents were quite different. The main common constituents of all the volatile oils were a-pinene, myrcene, a-humulene, β-caryophylene, spathulenol, and caryophyllene oxide. Chrysanthemum species are generally considered to be have a broad spectrum biologically activity, such as antioxidant, anticancer, aldose reductase inhibition;anti-inflammation, curing osteoporosis, antifungal activity and antimicrobial activities (Cheng et al. 2005; Cheon et al. 2009; Lee et al. 2009; Chang et al. 2010).
In the present study, chemical compositions of volatile oil obtained from C. morifolium were identified by GC–MS, the effect of surfactant, temperature, pH and UV on the antibacterial activity against several selected bacteria were evaluated too. It is aim to evaluate the valuable chemical materials, provide accurate compositional data as index of C. morifolium and provide a scientific basis for comprehensive development and utilization in C. morifolium.
Materials and methods
Materials
Chrysanthemum morifolium Ramat. Hemsl was picked in October 2015 from Baoxing County in Sichuan Province, China, and was supplied by Mao Yuan Agricultural Science and Technology Development Co., Ltd. The flowers dried at 40 °C for 12 h and was ground with a micro plant grinding machine to fine powder, then pulverized and sieved through a 0.15 mm sieve, sealed and set aside for further use.
Anhydrous acetone, Anhydrous sodium sulfate, Anhydrous alcohol, Agar powder (all of analytical grade) were purchased from Chengdu Kelong Chemical Reagent Co., Ltd. (Chengdu, China); Peptone, beef extract from Beijing Aoboxing Biotechnology Co., Ltd. (Beijing, China).
The antibacterial activities of the volatile oil were tested against Gram negative strain, Staphylococcus aureus ATCC 25923, Bacillus subtilis CICC 20612 and Gram positive strain. Escherichia coli ATCC 25922, Salmonella enteritids CICC 21482, Pseudomonas aeruginosa ATCC 27853. The strains were stored at − 80 °C in Microbiology Laboratory, College of Food Science, Sichuan Agricultural University.
Volatile oil extraction
30 g C. morifolium powder was placed in the volatile oil extractor, 400 mL distilled water was added, heated to reflux extraction for 9 h, dried with Na2SO4 to get Chrysanthemum volatile oil. In this study, hydro distillation extraction (HDE) method (Schultz et al. 1997) was used since it does not use organic solvents capable of contaminating the plant volatile oil.
It exactly weighed 0.80 g C. morifolium volatile oil and used anhydrous acetone to volume to 10 mL, over 0.45 μm organic membrane, took 1 mL to get on GC–MS determination.
Components analysis of volatile oils by GC–MS analysis
The analysis of the volatile oil was performed using Agilent 7890A-5975C GC–MS (Agilent technologies company in the United States), equipped with a HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm) for the separation. The mass-selective detector was operated in electron impact ionization (EI) mode with a mass scan range from m/z 40–550 at 70 eV. Helium (He) was used as the carrier gas at a flow rate of 1 mL/min. Injector and MS transfer line temperatures were set at 250 and 170 °C, respectively. A sample of 1.0 μL of volatile oil was injected manually using a 5:1 split ratio. The temperature program was as follows: initial temperature of 75 °C held for 2 min, followed by the ramping up of the temperature at a rate of 5 °C/min up to 130 °C, which was held for 7 min, 2.5 °C/min up to 170 °C for 10 min, finally 5 °C/min up to 250 °C for 10 min. The temperature of the MSD transfer line was 250 °C. The temperature of the ion source was 230 °C, and that of the MS quadrupole was 280 °C.
The components were identified by comparing their GC retention indices, NIST mass spectral search program (Version 2.0, National Institute of Standards and Technology), and mass spectra with published data. The compounds were tentatively identified on the basis of their retention times and by interpretation of MS fragmentation patterns with those of standard libraries NIST11. Amounts of the detected volatiles were based on comparison of their peak areas. One-way analysis of variance (ANOVA) and Duncan’s multiple range tests were carried out to compare significant differences (at the 5% significance level, p < 0.05) between treatments using the statistical package SPSS 20.0 for Windows.
Antibacterial activity analysis
Antibacterial tests carried out by the Oxford cup method using 200 μL of suspension containing 106 CFU/mL of bacteria spread on nutrient agar (NA) medium. Oxford cups (6 mm in diameter) were placed on the inoculated agar, and 100 μL of volatile oil was added into each Oxford cup. Ethanol solution (95%) was used as the negative control, placed for 2 h at 4 °C to completely diffused into the petri plates, then incubated at 37 °C for 24 h. The inhibition zone diameter was measured with a vernier. Tests were performed in triplicate.
Minimum inhibitory concentration (MIC) analysis
Volatile oils were added into the nutrient agar petri plates ranged at a concentration of 1.30, 0.67, 0.325, 0.163 and 0.081%, respectively. Test strain suspension was adjusted to 103–104 CFU/mL. For each test, a suspension of the tested strain (100 µL) was spread on the petri plates, and incubated at 37 °C for 24 h. The MIC is defined as the lowest concentration of the volatile oil at which the microorganism does not demonstrate visible growth on the plates.
Antibacterial stability analysis
Surfactants
Tween 20, Tween 80, and SDS were used to evaluate antibacterial activities of volatile oil dissolved in surfactants. The volatile oil for antibacterial tests was prepared with a concentration of 10% (v/v) solution filtered by through 0.22 μm filters. Antibacterial activities were measured according to the method described previously, Tween 20, Tween 80, and SDS surfactants as the control respectively.
pH
Volatile oil was diluted to a concentration of 10% (v/v) solution. Solution pH values were adjusted to 2, 4, 7, 9, 11 with 1 mol/L of NaOH or 1 mol/L of HCl respectively, and then solution was adjusted to near neutral pH 15 min later, and filtered by 0.22 μm filter. Antibacterial activities were measured according to the method above, 95% ethanol as a control.
Temperature
Volatile oil was dissolved in 95% ethanol to a concentration of 10% (v/v) solution and filtered by 0.22 μm Organic filter membrane, then placed at 40, 60, 80, 100 and 121 °C for 15 min, respectively. Antibacterial activities were tested according to the method above.
UV irradiation
Volatile oil was dissolved in 95% ethanol to a concentration of 10% (v/v) solution and filtered by 0.22 μm organic filter membrane. The irradiation was done by lamp with power of 8 W. The UV radiation with waves of 253.7 nm was performed from the distance of lamp to the sample of 40 cm. The following times of irradiation 10, 20, 30, 40 and 50 min were used, respectively. Antibacterial activity of volatile oil exposed to UV irradiation was determined with the method previously.
Results and discussions
Identification of chemical composition of C. morifolium volatile oil
The chemical composition of the volatile oil was analyzed by GC–MS, and the result is listed in Table 1. As presented in Table 1, fifty-six components were identified, constituting 42.00% of the volatile oil composition of C. morifolium. Hydrocarbons represented 32.00% in oil was the most abundant compound, followed by esters (2.03%), organic acids (3.65%), ketones (1.97%), alcohols (1.69%), aldehydes (0.62%), phenols (0.04%). The major components in the volatile oil of C. morifolium are monoterpenes and sesquiterpenes, including α-curcumene, α-farnesene, β-bisabolene, bisabolol, capric acid, linoleic acid, n-heptadecane, nonadecane and n-pentacosane. Among them the highest amount is α-curcumene (12.55%), this is consistent with the findings of Chang and Kim (2009). In the report, thirty-six volatile components of Chrysanthemum constituted 58.15% of the total volatile composition were characterized tentatively, consisting of 19 hydrocarbons, 7 alcohols, 2 ketones, 2 esters, 4 aldehydes, 1 oxide, and 1 miscellaneous component. The predominant components of Chrysanthemum were α-curcumene, and α-sesquiphellandrene. Whereas, α-pinene, 1,8-cineol, and chrysanthenone were the main aroma compounds of in Korea.
Table 1.
Chemical Composition of the volatile oil from C. morifolium
| No. | Constituent | Molecular formula | Molecular weight | Compositiona (%) |
|---|---|---|---|---|
| Hydrocarbon | 32.000 | |||
| 1 | 1,2,5,5-Tetramethyl-1,3-Cyclopentadiene | C9H14 | 122 | 0.023 |
| 2 | 2,4-Dimethyl Styrene | C10H12 | 132 | 0.035 |
| 3 | 2-Ethyl-p-Xylene | C10H14 | 134 | 0.023 |
| 4 | Ionene | C13H18 | 174 | 0.144 |
| 5 | α-Cedrene | C15H24 | 204 | 0.121 |
| 6 | β-Caryophyllene | C15H24 | 204 | 0.269 |
| 7 | β-Farnesene | C15H24 | 204 | 0.582 |
| 8 | (−)-Isocaryophyllene | C15H24 | 204 | 0.278 |
| 9 | γ-selinene | C15H24 | 204 | 0.117 |
| 10 | α-Curcumene | C15H22 | 202 | 12.545 |
| 11 | α-farnesene | C15H24 | 204 | 3.443 |
| 12 | β-Bisabolene | C15H24 | 204 | 1.029 |
| 13 | 7-α-Selinene | C15H24 | 204 | 0.724 |
| 14 | β-Sesquiphellandrene | C15H24 | 204 | 0.871 |
| 15 | 3-Octadecene | C18H36 | 252 | 0.092 |
| 16 | 1,2,3,4-Tetrahydro-1,6,8-Trimethylnaphthalene | C13H18 | 156 | 0.077 |
| 17 | 1,2,3,4-Tetrahydro-1,5,7-Trimethylnaphthalene | C13H18 | 156 | 0.061 |
| 18 | 1,1,6-Trimethyl-1,2-Dihydronaphthalene | C13H16 | 154 | 0.349 |
| 19 | Cyclopentadecane | C15H30 | 210 | 0.128 |
| 20 | n-Heptadecane | C17H36 | 240 | 3.185 |
| 21 | Octadecane | C18H38 | 254 | 0.142 |
| 22 | Nonadecane | C19H40 | 268 | 4.179 |
| 23 | n-Eicosane | C20H42 | 282 | 0.103 |
| 24 | n-Heneicosane | C21H44 | 296 | 0.060 |
| 25 | 2-Methyleicosane | C21H44 | 296 | 0.117 |
| 26 | n-Docosane | C22H46 | 310 | 0.491 |
| 27 | n-Tetracosane | C24H50 | 338 | 0.251 |
| 28 | n-Pentacosane | C25H52 | 352 | 2.024 |
| 29 | 10-Methylicosane | C21H44 | 296 | 0.163 |
| 30 | n-Heptacosane | C27H56 | 380 | 0.374 |
| Esters | 2.034 | |||
| 31 | Phthalic Acid-Butyl Tetradecyl Ester | C26H42O4 | 418 | 0.130 |
| 32 | Dibutyl Phthalate | C16H22O4 | 278 | 0.061 |
| 33 | Ethyl Palmitate | C18H36O2 | 284 | 0.397 |
| 34 | Methyl Linoleate | C19H34O2 | 294 | 0.235 |
| 35 | Acetic Acid-3,7,11,15-Tetramethyl-Hexadecyl Ester | C22H44O2 | 340 | 0.342 |
| 36 | Ethyl Linoleate | C20H36O2 | 306 | 0.404 |
| 37 | Ethyl Linolenate | C20H34O2 | 306 | 0.330 |
| 38 | Di(2-Ethylhexyl) Phthalate | C24H38O4 | 390 | 0.060 |
| 39 | L-Bornyl Acetate | C12H20O2 | 292 | 0.075 |
| Aldehydes | 0.616 | |||
| 40 | Isocyclocitral | C10H16O | 152 | 0.565 |
| 41 | P-Isopropylbenzaldehyde | C10H12O | 148 | 0.009 |
| Phenols | 0.042 | |||
| 42 | 2,2′-Methylene Bis (6-Tert-Butyl-4-Methyl)Phenol | C23H32O2 | 340 | 0.042 |
| Alcohols | 1.689 | |||
| 43 | (−)-Terpinen-4-ol | C10H18O | 154 | 0.033 |
| 44 | 2-Methyl-5-(1-Methyl Ethenyl)-2-Cyclohexen-1-ol | C10H16O | 152 | 0.021 |
| 45 | Bisabolol | C15H26O | 222 | 1.177 |
| 46 | Isophytol | C20H40O | 296 | 0.067 |
| 47 | Phytol | C20H40O | 296 | 0.391 |
| Ketones | 1.974 | |||
| 48 | Camphor | C10H16O | 152 | 0.02 |
| 49 | Phytone | C18H36O | 268 | 0.279 |
| 50 | 2-Heptadecanone | C17H34O | 254 | 0.219 |
| 51 | 2-methylene-5-(1-methylethyl)cyclohexanone | C16H28O2 | 252 | 0.282 |
| 52 | 1-Tert-Butyl-7-Methoxynaphthalene | C15H18O | 214 | 1.174 |
| Organic acids | 3.646 | |||
| 53 | Capric Acid | C10H20O2 | 172 | 1.005 |
| 54 | Palmitic Acid | C16H32O2 | 256 | 0.572 |
| 55 | Linoleic Acid | C18H32O2 | 280 | 2.028 |
| 56 | β-Acetylacrylic Acid | C5H6O3 | 114 | 0.041 |
| Total | 42.000 |
aPercentage of relative peak aera
Antibacterial activity of C. morifolium volatile oil
Chrysanthemum morifolium volatile oil is liquid at room temperature and does not dissolve in water. In the experiments, volatile oil can be dissolved in ethyl acetate, 95% ethanol, isopropyl alcohol, acetone, n-octanol, n-hexane and n-heptane. But the above organic solvents had certain suppression bacterial effect for tested strains. The pre-test results showed that 95% ethanol’s inhibition effect on tested strains is relatively weak, so 95% ethanol was used as solvent for volatile oil. The inhibitory effects are presented in Fig. 1.
Fig. 1.
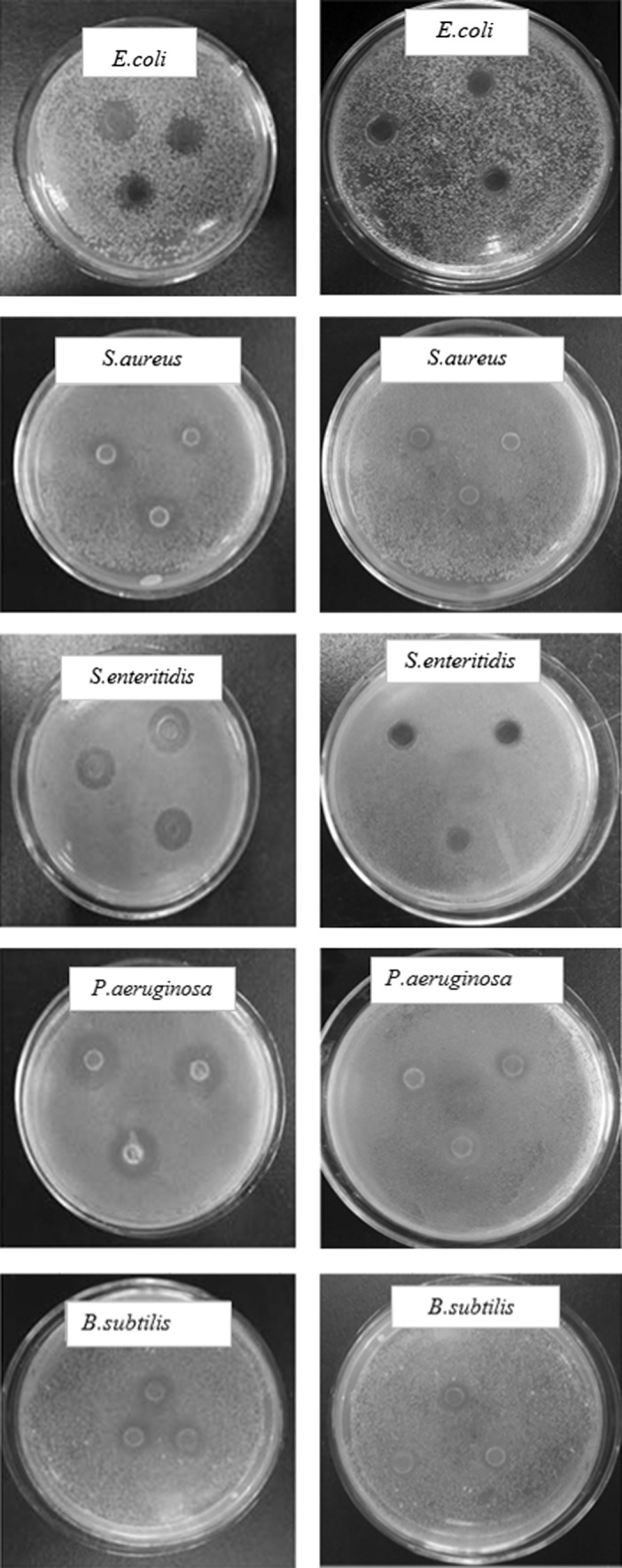
the inhibitory effect of the Chrysanthemum morifolium R. volatile oil on 5 kinds of tested microbes, on the left is the experimental group, the right is the control group (95% ethanol)
In Table 2, it had a certain inhibitory effect on the test strains and the inhibition zone diameter varied from 7.64 to 8.51 mm. 10% volatile oil solution diluted by 95% of ethanol had obvious inhibitory effect on E. coli, S. aureus, S. enteritidis, P. aeruginosa and B. subtilis. Wherein the maximum inhibition was P. aeruginosa and inhibition zone diameter was 20.43 mm. The minimum inhibitory effect was E. coli and inhibition zone diameter is 12.29 mm, which may be the result of the inhibitory effect of ethanol and volatile oil synergy.
Table 2.
The inhibitory effect of C. morifolium. volatile oil on E. coli, S. aureus, S. enteritidis, P. aeruginosa, B. subtilis 5 kinds of test bacteria and the minimum inhibitory concentration
| Microorganisms | Inhibition zone diameter (mm) | MIC | |
|---|---|---|---|
| 10% Volatile oil of Chrysanthemum morifolium R. | Control | Concentration (v/v) (%) | |
| E. coli | 12.29 ± 0.49 | 8.51 ± 0.61 | 1.30 |
| S. aureus | 13.14 ± 0.60 | 7.64 ± 0.43 | 1.30 |
| S. enteritidis | 15.20 ± 0.64 | 8.26 ± 0.13 | 0.67 |
| P. aeruginosa | 20.43 ± 0.69 | 8.38 ± 0.23 | 0.33 |
| B. subtilis | 14.16 ± 0.54 | 8.34 ± 0.41 | 0.67 |
Through analysis of variance, the sample group showed significant difference compared with the untreated group (p < 0.01) in inhibitory effect on 5 tested strains. It showed that volatile oil inhibited 5 tested strains. The inhibition size ranked P. aeruginosa > S. enteritidis > B. subtilis > S. aureus > E. coli. The plant volatile oils are tremendous enriched with terpenoids which exert inhibitory action against microorganisms by disrupting their membranes (Burt 2004). In the present work, the main components of C. morifolium volatile oil are monoterpenes and sesquiterpenes, including α-curcumene, α-farnesene, β-bisabolene, bisabolol, capric acid, linoleic acid, n-heptadecane, nonadecane and n-pentacosane. The antibacterial activity of the oils could, in part, be associated with α-curcumene, which was previously reported for its antibacterial activity (Schwob et al. 2002). In addition, the components in lower amount such as β-bisabolene and bisabolol, which are already known to exhibit antibacterial activity (Forrer et al. 2013; Kamatou and Viljoen 2010), could also contribute to the antibacterial activity of the oils.
The MIC of volatile oil against 5 tested strains showed that minimum inhibitory concentration of E. coli and S. aureus was 1.30%, S. enteritidis and B. subtilis was 0.67%, P. aeruginosa was 0.32%. Currently, the antibacterial mechanism of volatile oil is not very sure. Therefore, the further studies need to explore the components of the volatile oil which play a key role in the antibacterial effect. Özcan and Erkmen (2001) analyzed the antimicrobial activity of the essential oil of nine plant spices. The results showed that the essential oil tested varied in their antimicrobial activity. Individual or combinations of plant essential oils may provide an efficacious mixture for the inactivation of pathogenic and spoilage microorganisms, and to achieve adequate shelf-life of foods. Hu et al. (2009) analyzed the volatile oils from the flowers, leaves, barks, roots and fruits of A. brachypus were extracted individually by hydro distillation, and their chemical constituents were isolated and characterized by means of GC–MS. The antimicrobial activities of the volatile oils was evaluated against 11 microorganisms (9 strains bacteria and 2 strains yeast) using agar disc diffusion and broth micro-dilution methods. The bacteria, including gram-positive bacteria and gram-negative bacteria, were more sensitive to the oils than yeasts.
Antibacterial activity stability of volatile oil
Volatile oils are particularly prone to quantitative and quantitative change due to environmental factors. In consideration of changes of volatile oil which may influence antibacterial activity. The effect of environmental factors including surfactant, temperature, pH and ultraviolet was showed.
Surfactants
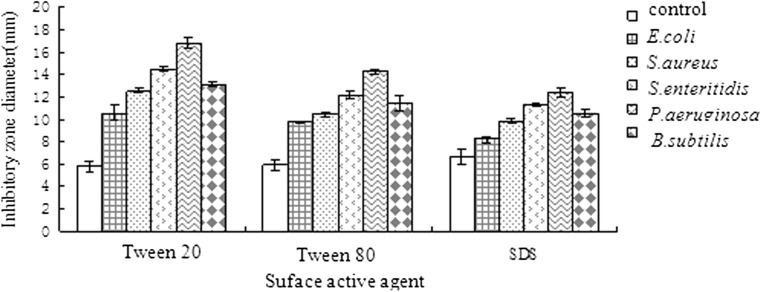
In Fig. 2, volatile oil dissolved in different surfactants showed significant activity against selected strains. The inhibition zone diameter against was E. coli, S. aureus, S. enteritidis, P. aeruginosa and B. subtilis were 8.17 to 10.57 mm, 9.81 to 12.52 mm, 11.32 to 14.47 mm, 12.34 to 16.77 mm and 10.48 to 13.12 mm respectively. The variance analysis showed that the antibacterial activity of volatile oil dissolved in surfactants against five bacteria strains was significant (p < 0.01). Inhibition zone diameters were less than 7 mm in untreated group and there is no inhibitory effect. Comprehensive above, with 10% Tween 20, Tween 80 solution and 5% SDS solution prepared 10% volatile oil solution had inhibitory effect. When volatile oil dissolved in Tween 20 had the strongest antibacterial activity, followed by Tween 80 and 5% SDS solution. This is mainly due to the Tween 20 and Tween 80 as emulsifier allow volatile oil to solubilize effective antibacterial ingredient to the solution. But the volatile oil solution of Tween 80 formulated more viscous, not conducive to the spread which results that volatile oil dissolved in Tween 20 is better than in Tween 80. Volatile oil dissolved in SDS solution had weaker solubilization and emulsification than Tween 20 and Tween 80. Therefore, the C. morifolium volatile oil antibacterial active ingredient can not distribute evenly in the solution and the SDS solution had the weakest antibacterial effect.
Fig. 2.
Effect of different surfactants on the antibacterial activity of Chrysanthemum morifolium R. volatile oil
Temperature
In Table 3, Inhibition zone diameters of C. morifolium volatile oil treated at different temperatures on E. coli was range from 11.81 to 12.40 mm with untreated group 12.21 mm. S. aureus were 12.96 to 13.32 mm with untreated group 13.01 mm, S. enteritidis were 15.04 to 15.30 mm with untreated group 15.18 mm. P. aeruginosa’s were 19.63 to 20.12 mm with untreated group 19.92 mm. B. subtilis were 13.96 to 14.42 mm with untreated group 14.06 mm. No significant differences was detected among 5 tested strains on C. morifolium volatile oil treated at different temperature by analysis of variance temperature (p > 0.05). LSD multiple comparisons showed that the difference between the treatment group and untreated group was not significant, too. Briefly, heat treatment had no effect on antibacterial stability of C. morifolium volatile oil, may be attributed to the main antibacterial ingredient, such as terpene compounds, aldehyde and ketone compounds, acids, alcohols, phenols and other substances were not decomposed. As a result, this result forecast that C. morifolium volatile oil may be a potential volatile oil as natural preservatives added in food and it can still play a role in antimicrobial preservative in the course of food processing.
Table 3.
Effect of different temperature, pH, UV on the antibacterial activity of C. morifolium volatile oil inhibition zone diameter:mm
| Microorganisms | Temperature | Untreated | ||||
|---|---|---|---|---|---|---|
| 40 °C | 60 °C | 80 °C | 100 °C | 121 °C | ||
| E. coli | 12.34 ± 0.45 | 12.40 ± 0.15 | 12.08 ± 0.30 | 12.18 ± 0.36 | 12.01 ± 0.23 | 12.21 ± 0.25 |
| S. aureus | 13.12 ± 0.26 | 13.32 ± 0.45 | 13.09 ± 0.27 | 13.05 ± 0.28 | 12.96 ± 0.22 | 13.01 ± 0.32 |
| S. enteritidis | 15.23 ± 0.22 | 15.22 ± 0.21 | 15.04 ± 0.19 | 15.30 ± 0.28 | 15.14 ± 0.07 | 15.18 ± 0.25 |
| P. aeruginosa | 20.06 ± 0.35 | 19.91 ± 0.20 | 19.63 ± 0.63 | 20.12 ± 0.25 | 20.04 ± 0.30 | 19.92 ± 0.42 |
| B. subtilis | 14.42 ± 0.18 | 14.06 ± 0.39 | 13.94 ± 0.31 | 14.16 ± 0.38 | 13.96 ± 0.30 | 14.06 ± 0.36 |
| Microorganisms | pH | Untreated | ||||
|---|---|---|---|---|---|---|
| 2 | 4 | 7 | 9 | 11 | ||
| E. coli | 11.38 ± 0.24 | 11.55 ± 0.24 | 12.08 ± 0.48 | 12.18 ± 0.23 | 11.54 ± 0.19 | 12.15 ± 0.23 |
| S. aureus | 12.22 ± 0.50 | 12.74 ± 0.26 | 13.09 ± 0.24 | 12.84 ± 0.19 | 12.79 ± 0.19 | 12.92 ± 0.26 |
| S. enteritidis | 14.06 ± 0.19 | 14.42 ± 0.32 | 15.13 ± 0.32 | 14.85 ± 0.29 | 13.80 ± 0.47 | 15.22 ± 0.15 |
| P. aeruginosa | 19.36 ± 0.44 | 19.89 ± 0.14 | 20.11 ± 0.15 | 20.01 ± 0.23 | 19.37 ± 0.39 | 20.08 ± 0.35 |
| B. subtilis | 13.22 ± 0.26 | 13.83 ± 0.31 | 14.12 ± 0.39 | 13.91 ± 0.14 | 13.01 ± 0.21 | 14.06 ± 0.24 |
| Microorganisms | UV | Untreated | ||||
|---|---|---|---|---|---|---|
| 10 min | 20 min | 30 min | 40 min | 50 min | ||
| E. coli | 12.09 ± 0.45 | 11.91 ± 0.35 | 12.22 ± 0.06 | 12.03 ± 0.16 | 11.98 ± 0.27 | 12.15 ± 0.22 |
| S. aureus | 13.08 ± 0.30 | 13.14 ± 0.26 | 13.12 ± 0.28 | 13.18 ± 0.30 | 13.07 ± 0.22 | 13.09 ± 0.28 |
| S. enteritidis | 15.18 ± 0.39 | 14.92 ± 0.23 | 15.09 ± 0.14 | 14.95 ± 0.23 | 14.88 ± 0.28 | 15.21 ± 0.32 |
| P. aeruginosa | 19.68 ± 0.65 | 19.97 ± 0.16 | 20.10 ± 0.14 | 19.90 ± 0.20 | 19.96 ± 0.15 | 20.04 ± 0.38 |
| B. subtilis | 14.02 ± 0.39 | 13.86 ± 0.35 | 13.51 ± 0.23 | 13.68 ± 0.55 | 13.65 ± 0.47 | 13.78 ± 0.24 |
pH
In Table 3, C. morifolium volatile oil solution was treated with different pH, E. coli inhibition zone diameters was ranged from 11.38 to 12.18 mm with untreated group 12.15 mm. The significantly different among volatile oil’s antibacterial activity treated by different pH was show by variance analysis (p < 0.01). LSD multiple comparison showed that no significant treatment between treated groups pH 7–9 and control groups. While other treated groups and untreated group were significantly different. The inhibition zone diameters on S. aureus was ranged from 12.22 to 13.09 mm with untreated group 12.92 mm. Variance analysis showed that the C. morifolium volatile oil handled by different pH showed significantly different antibacterial activity (p < 0.05). LSD multiple comparison showed that, pH 2 treatment group and untreated group was highly significant, while other treatments and untreated groups were not significantly different. Inhibition zone diameter against S. enteritidi were 13.80–15.13 mm with untreated group 15.22 mm. The significantly different antibacterial activity among treated groups was detected too by variance analysis (p < 0.01). LSD multiple comparisons showed, treatment group pH 7–9 and untreated group was not significant difference but other treatments and with untreated group were significantly different. P. aeruginosa’s inhibition zone diameters was within the range of 19.36–20.11 mm with untreated group 20.08 mm. Variance analysis showed that volatile oil’s antibacterial activity handled by different pH was significantly different (p < 0.05) and LSD multiple comparison showed treatment groups (pH 4, pH 7 and pH 9) and untreated groups were not significant different. B. subtilis’s inhibition zone diameters was within the range of 13.01–14.12 mm while untreated showed 14.06 mm. Variance analysis showed that the antibacterial activity vary significantly by different pH treatment (p < 0.01). LSD multiple comparisons showed that under pH 4, pH 7 and pH 9 conditions, the difference between treatment group and untreated group was not significant. However, the other treatments and the untreated group were significantly different. In summary, acid and alkali environment had effect on C. morifolium volatile oil to weaken the inhibitory effect. In the near neutral environment, the impact on C. morifolium volatile oil inhibitory effect was not obvious.
UV
In Table 3, C. morifolium volatile oil antibacterial solution was processed through ultraviolet irradiation at different times. The inhibition zone diameters of E. coli of volatile oil ranged from 11.91 to 12.22 mm, while the untreated group was 12.15 mm. Then, the inhibition zone diameters of S. aureus’s ranged from 13.07 to 13.18 mm, and the untreated group was 13.09 mm. Moreover, the inhibition zone diameters of S. enteritidis’s ranged from 14.88 to 15.18 mm, and the untreated group was 15.21 mm. In addition, the inhibition zone diameters of P. aeruginosa ranged from 19.68 to 20.10 mm, and the untreated group was 20.04 mm. B. subtilis’s ranged from 3.51 to 14.02 mm, and the untreated group was 13.78 mm. Result of variance analysis showed that it is no significant differences antibacterial activity of C. morifolium volatile oil to 5 tested strains (p > 0.05) under the ultraviolet irradiation at different times. LSD multiple comparison indicted that the treatment group and the untreated group were not significant. In summary, the UV light irradiation had little influence on the stability of C. morifolium volatile oil antibacterial activity.
Conclusion
The research identified 56 kinds of compounds from C. morifolium volatile oil, the main components were monoterpenes and sesquiterpenes compounds, including hydrocarbons, esters, aldehydes, ketones, phenols and organic acids. Among them, α-curcumene had the highest proportion, accounting for 12.55%. C. morifolium volatile oil had certain inhibitory effects on 5 tested strains and the inhibitory effects ranked: P. aeruginosa > S. enteritidis > B. subtilis > S. aureus > E. coli. Wherein it had the maximum inhibitory effect on P. aeruginosa and inhibition zone diameter reached 20.43 mm. The minimum inhibitory effect on E. coli and its inhibition zone diameter was 12.29 mm. Surfactant had great impact on its antibacterial stability and C. morifolium volatile oil had strongest antibacterial activity with Tween 20 as emulsifier and Tween 80 as emulsifier followed and SDS was weakest. The pH has certain influence on the stability antibacterial activity of C. morifolium volatile oil. Temperature and UV had the least influence on the stability of antibacterial activity.
Acknowledgements
This project is financially supported by Scientific Foundation of Baoxin County for Chrysanthemum industry development, Sichuan Province.
References
- Burt S. Essential oils: their antibacterial properties and potential applications in food-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chang KM, Kim GH. Comparative chemical composition of domestic and imported Chrysanthemum indicum L. flower oils. Food Sci Biotechnol. 2009;18(5):1288–1292. [Google Scholar]
- Chang KM, Kim GH. Volatile aroma constituents of gukhwa (Chrysanthemum morifolium R.) Food Sci Biotechnol. 2013;22(3):659–663. doi: 10.1007/s10068-013-0128-3. [DOI] [Google Scholar]
- Chang KM, Choi EM, Kim GH. Chemical constituents of Chrysanthemum indicum L. flower oil and effect on osteoblastic MC3T3-E1 cells. Food Sci Biotechnol. 2010;19(3):815–819. doi: 10.1007/s10068-010-0114-y. [DOI] [Google Scholar]
- Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J Ethnopharmacol. 2005;101(1):334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Cheon MS, Yoon TS, Lee DY, Choi GY, Moon BC, Lee AY, Choo BK, Kim HK. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-κB and MAPKs activation in lipopolysaccaride-induced RAW 264.7 macrophages. J Ethnopharmacol. 2009;122:473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Choi HS, Kim GH. Volatile flavor composition of gamguk (Chrysanthemum indicum) flower essential oils. Food Sci Biotechnol. 2011;20(2):319–325. doi: 10.1007/s10068-011-0045-2. [DOI] [Google Scholar]
- Enan E. Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol. 2001;130:325–327. doi: 10.1016/s1532-0456(01)00255-1. [DOI] [PubMed] [Google Scholar]
- Forrer M, Kulik EM, Filippi A, Waltimo T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a gram-positive bacterium associated with halitosis. Arch Oral Biol. 2013;58(1):10–16. doi: 10.1016/j.archoralbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Haouas D, Cioni PL, Halima-Kamel MB, Flamini G, Hamouda MHB. Chemical composition and bioactivities of three Chrysanthemum essential oils against Tribolium confusum (du Val) (Coleoptera: Tenebrionidae) J Pest Sci. 2012;85(3):367–379. doi: 10.1007/s10340-012-0420-7. [DOI] [Google Scholar]
- Hu HB, Zheng XD, Hu HS, Li Y. Chemical compositions and antimicrobial activities of essential oils extracted from Acanthopanax brachypus. Arch Pharm Res. 2009;32(5):699–710. doi: 10.1007/s12272-009-1508-3. [DOI] [PubMed] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Kamatou GP, Viljoen AM. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc. 2010;87(1):1–7. doi: 10.1007/s11746-009-1483-3. [DOI] [Google Scholar]
- Lee DY, Choi GY, Yoon TS, Cheon MS. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;123:149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M. Medicinal flowers. vi. absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull. 2002;50(7):972–975. doi: 10.1248/cpb.50.972. [DOI] [PubMed] [Google Scholar]
- Özcan M, Erkmen O. Antimicrobial activity of the essential oils of Turkish plant spices. Eur Food Res Technol. 2001;212(6):658–660. doi: 10.1007/s002170100310. [DOI] [Google Scholar]
- Schultz TH, Flath RA, Mon TR, Eggling SB, Teranishi R. Isolation of volatile components from a model system. J Agric Food Chem. 1997;25:446–449. doi: 10.1021/jf60211a038. [DOI] [Google Scholar]
- Schwob I, Bessiere JM, Dherbomez M, Viano J. Composition and antimicrobial activity of the essential oil of Hypericum coris. Fitoterapia. 2002;73(6):511–513. doi: 10.1016/S0367-326X(02)00171-5. [DOI] [PubMed] [Google Scholar]
- Wu XN, Yu CH, Bao QN. And the mechanism of anti-inflammatory effects of total flavonoids of Chrysanthemum morifolium Ramat. J Clin Pharmacol Ther China. 2009;14(9):1000–1003. [Google Scholar]