Figure 1.
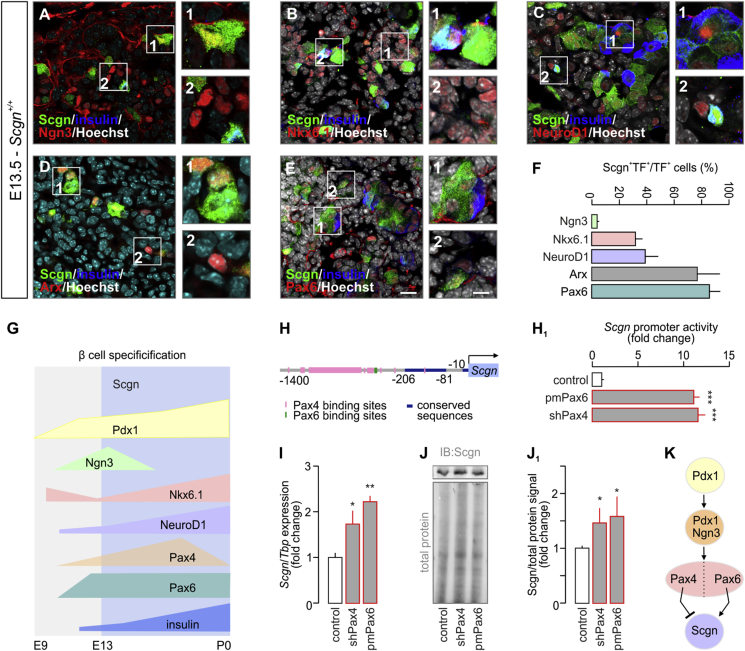
Pax4 and Pax6 control secretagogin levels during embryonic pancreas development. (A–E) Co-immunolabeling of secretagogin (Scgn) and transcription factors crucial for the development of the endocrine pancreas in E13.5 mouse fetuses. Secretagogin was detected in a subset of Ngn3+ cells even if the majority of Ngn3+ cells did not co-express Scgn (A). Notably, most secretagogin+ progenitors belonged to the pool of either Nkx6.1+ (B) or NeuroD1+ (C) cells with more than half of Nkx6.1+/NeuroD1+ progenitors being Scgn− (B, C). Arx, marking α cell fate, was largely co-expressed with Scgn (D). Likewise, Scgn immunoreactivity was found in almost all Pax6+ cells (E). Hoechst 33,342 (pseudo-coloured in grey) was used as nuclear counterstain. Scale bars = 10 μm or 4 μm (for numbered inserts). Representative images are shown. (F) Quantitative analysis of Scgn co-expression with Ngn3, Nkx6.1, NeuroD1, Arx and Pax6 in embryonic pancreas. The percentage ratio of Scgn+ and transcription factor (TF)+ dual-labelled cells was expressed by using the total number of TF+ cells as denominator. n > 100 cells from n = 3 pancreata were counted. (G) Schematic timeline of TF levels crucial for β cell specification. Cell differentiation, maturation, and maintenance of β cell identity are underpinned by the transcriptional activity of Nkx6.1, NeuroD1, Pax4, and Pdx1 [6]. Pancreas development commences at ∼ E9 with expression of Pdx1 in pancreatic progenitors [1]. Subsequently, Pdx1-driven transient presence of Ngn3 promotes development of endocrine progenitors [2]. While Nkx6.1 expression represses pre-acinar cell fate [33], Ngn3-dependent NeuroD1 activity facilitates of the differentiation of pancreatic endocrine cells [3]. This is then followed by Ngn3-and Pdx1-mediated expression of Nkx6.1, Pax6, and Pax4[4], [5], [6], [7], [8]. Coincident Pdx1, NeuroD1, Nkx6.1, Pax4, and Pax6 transcriptional activity establishes the cohort of pancreatic endocrine cells, which co-express glucagon and insulin [9]. High Pax4 activity represses glucagon expression, thus promoting β cell fate [10]. Secretagogin (Scgn) appears in the endocrine pancreas at the beginning of the secondary transition (∼E13), when α vs. β cell fate decisions are dictated by TFs. (H-H1) In silico prediction of Pax4 and Pax6 TF binding sites within the murine secretagogin promoter (up to −1,400 bps). (H1) Overexpression of Pax6 (pmPax6), as well as Pax4 knock-down (shPax4) significantly increase Scgn promoter activity in INS-1E cells. Promoter activity was defined as a ratio of firefly-to-Renilla luciferase chemiluminescence. (I) Pax6 overexpression and shRNA-mediated silencing of Pax4 (shPax4) increase Scgn mRNA levels in vitro. TATA-binding protein (Tbp), a house keeping gene, was used to normalize gene expression. (J–J1) Pax6 overexpression and shPax4 increase secretagogin protein content in INS-1E cells. Quantitative data reflect fold changes that had been normalized to total protein content. (K) Schema of the upstream regulation of secretagogin (Scgn) expression. Pdx1 initially expressed in pancreatic progenitors drives Ngn3 expression to promote differentiation of pancreatic endocrine progenitors. Subsequent expression and opposing actions of Pax4 and Pax6 in these cells control Scgn level. Representative images and immunoblots are shown. Data were expressed as means ± s.d. from triplicate experiments. ***p < 0.001, **p < 0.01, *p < 0.05 calculated with pair-wise comparisons/one-way ANOVA.