Graphical abstract
Keywords: Transacatheter aortic valve replacement, Thrombosis, Anticoagulation
Highlights
-
•
A case of very severe transcatheter heart valve thrombosis is described.
-
•
The patient was treated with warfarin with success.
-
•
The first line of treatment of transcatheter heart valve thrombosis is anticoagulation.
-
•
A trial of 2-3 months of warfarin should be tried before considering alternatives.
Introduction
Transcatheter heart valve (THV) thrombosis is a rare condition. Data on the appropriate management of severe cases are scarce. We report a case of severe thrombosis of a SAPIEN XT valve (Edwards Lifesciences, Irvine, CA) successfully treated with anticoagulation.
Case Presentation
An 82-year-old man underwent transfemoral transcatheter aortic valve replacement (TAVR) for severe symptomatic aortic stenosis with a 29-mm SAPIEN XT prosthesis with excellent results. On postoperative day 2, the patient suffered an anteroseptal ST-segment elevation myocardial infarction and was treated with a drug-eluting stent to the proximal left anterior descending artery. A week later, a stroke secondary to an apical thrombus occurred.
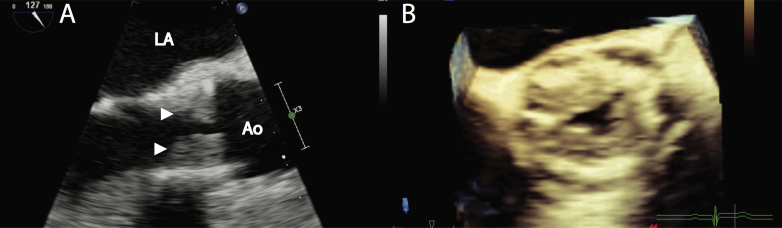
Eighteen months after TAVR, while on aspirin monotherapy, the patient consulted for recent-onset exertional angina. A loud late-peaking systolic aortic murmur with a decreased second heart sound was heard. Transthoracic echocardiography revealed a dense mobile mass measuring 1.5 cm in the left ventricular outflow tract, presumably attached to the prosthesis, associated with severely elevated mean gradient (71 mm Hg; dimensionless velocity index, 0.17; Figure 1A and Figure 2A; Video 1). A transesophageal echocardiogram confirmed the presence of a large burden of hypoechogenic material consistent with thrombus diffusely apposed to the ventricular side of the leaflets (Figure 3A, arrowheads; Video 2) resulting in severe opening restriction (aortic valve area by three-dimensional guided planimetry = 0.44 cm2; Figure 3B; Video 3).
Figure 1.
Two-dimensional still frames of THV thrombosis. (A) TTE apical five-chamber view at baseline. There is a mass in the left ventricular outflow tract (arrow). (B) TTE at 3-month follow-up. Magnified apical five-chamber view showing resolution of the mass in the left ventricular outflow tract. Ao, Aorta; LV, left ventricle; TTE, transthoracic echocardiography.
Figure 2.
Transprosthetic gradients. (A) TTE at baseline. Continuous-wave Doppler in the apical five-chamber view showing very high transprosthetic gradients (peak gradient, 104 mm Hg; mean gradient, 71 mm Hg). (B) TTE at 3-month follow-up. Continuous-wave Doppler in the apical five-chamber view showing almost complete resolution of high transprosthetic gradients, with a residual mean gradient of 15 mm Hg. TTE, Transthoracic echocardiography.
Figure 3.
Echocardiographic images of THV thrombosis (A) TEE. Two-dimensional midesophageal view of the aortic valve at 120° confirmed a large burden of hypoechogenic material consistent with thrombus diffusely apposed to the ventricular side of the leaflets (arrowheads). (B) Three-dimensional midesophageal short-axis view of the prosthetic aortic valve showed severely reduced opening. TEE, Transesophageal echocardiography. Ao, aorta.
After 8 days of systemic anticoagulation (intravenous heparin and then warfarin, international normalized ratio 2.0-3.0), the patient remained clinically stable but the gradient was unchanged. While surgical aortic valve replacement was contemplated, it was the consensus of the heart team to first attempt medical management with anticoagulation in addition to the low-dose aspirin. At 3-month follow-up, the control transthoracic echocardiogram showed resolution of the mass in the left ventricular outflow tract and near normalization of transprosthetic gradients (mean gradient, 15 mm Hg; Figure 1B, Figure 2B; Video 4), with resolution of symptoms. This patient will remain on lifelong warfarin and low-dose aspirin.
Discussion
Clinically significant THV thrombosis is a rare complication, and its incidence is uncertain.1 To date, most studies describing THV thrombosis are case series and retrospective reports. Notably, no case of THV thrombosis occurred in the PARTNER and Corevalve trials.2, 3, 4 A 2015 systematic review found a total of 11 publications with 16 patients (median time, 6 months post-TAVR).5 Out of the 16 patients, 13 received warfarin, which restored the mean transprosthetic gradient to baseline within 2 months in all, while three patients were treated with surgery.
Subsequently, Latib et al. published in 2015 the largest retrospective study with an incidence of TVH thrombosis of 0.6% (26 over 4,266 TAVR patients; median time to THV thrombosis, 181 days, all within 2 years from TAVR; mean gradient, 40.5 ± 14 mm Hg).6 Twenty-three patients were treated with anticoagulation, resulting in restoration of normal THV function in all within 2 months.
In a study of 405 TAVR patients, Hansson et al. reported 28 (7%) cases of THV thrombosis detected by contrast-enhanced multidetector computed tomography.7 Of these, 23 were subclinical and five presented as clinically overtly obstructive (1% of the cohort for the latter, all within 1 year from TAVR; mean gradient, 21 ± 6 mm Hg). Four out of these five patients were treated with anticoagulation, three with success and one patient died at follow-up, however, the cause of death was not specified and there was no follow-up imaging study data so it is unclear whether that patient failed to respond to anticoagulation.
While clinically significant THV thrombosis seems to be a rare event, multidetector computed tomography studies in patients after TAVR implantation such as the report from Hansson et al. have shown a high rate of subclinical thrombosis, ranging from 4% to 40%.7, 8, 9, 10 Patients taking warfarin appear to be at lower risk, and treatment with warfarin results in resolution in 81%-100% of cases.7, 8, 9, 10 The majority of patients with subclinical thrombosis are asymptomatic and have similar or only slightly increased transprosthetic gradients, so the clinical significance of subclinical thrombosis diagnosed by multidetector computed tomography is uncertain. In one report, subclinical thrombosis was possibly linked with higher stroke events.9 The best anticoagulation/antiplatelet regimen after TAVR is unknown. The ongoing GALILEO (NCT02556203, which compares rivaroxaban + aspirin for 3 months followed by rivaroxaban alone vs aspirin + clopidogrel for 3 months followed by aspirin alone) and POPular-TAVI trials (NCT02247128, which compares aspirin monotherapy to dual antiplatelet therapy in the first 3 months followed by aspirin monotherapy in both groups) will help to elucidate this important clinical question.
We present a unique case of late THV thrombosis with extremely high gradients and large diffuse thrombotic leaflet apposition. This case is unusual as it was particularly severe. This patient failed to improve after 1 week of anticoagulation; however, he ultimately showed good response to anticoagulation after 3 months, avoiding the need for a high-risk open-heart surgery. Scarce data exist on the expected early response to anticoagulation.
Conclusions
We report on a patient with late THV thrombosis and very severe prosthetic obstruction that ultimately showed resolution after 3 months of warfarin therapy. Systemic anticoagulation remains the treatment of choice for THV thrombosis. A trial of 2-3 months of systemic anticoagulation should be tried before considering failure to treatment. To our knowledge, there is no published case of clinically significant THV thrombosis that failed to respond to anticoagulation. THV thrombosis should be suspected in any patient with THV and unexplained elevated transprosthetic gradients, particularly within 2 years of implantation.
Footnotes
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.01.011.
Supplementary Data
Transthoracic echocardiography. Apical five-chamber view showing a mass in the left ventricular outflow tract.
Transesophageal echocardiography. Two-dimensional midesophageal view of the aortic valve at 120° confirmed a large burden of hypoechogenic material consistent with thrombus diffusely apposed to the ventricular side of the leaflets.
Transesophageal echocardiography. Three-dimensional midesophageal short-axis view of the prosthetic aortic valve showed severely reduced opening.
Transthoracic echocardiography at 3-month follow-up. Zoomed apical five-chamber view showing resolution of the mass in left ventricular outflow tract.
References
- 1.Dangas G.D., Weitz J.I., Giustino G., Makkar R., Mehran R. Prosthetic heart valve thrombosis. J Am Coll Cardiol. 2016;68:2670–2689. doi: 10.1016/j.jacc.2016.09.958. [DOI] [PubMed] [Google Scholar]
- 2.Kapadia S.R., Leon M.B., Makkar R.R., Tuzcu E.M., Svensson L.G., Kodali S. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Smith C.R., Miller D.C., Moses J.W., Tuzcu E.M. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 4.Reardon M.J., Adams D.H., Kleiman N.S., Yakubov S.J., Coselli J.S., Deeb G.M. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113–121. doi: 10.1016/j.jacc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Cordoba-Soriano J.G., Puri R., Amat-Santos I., Ribeiro H.B., Abdul-Jawad Altisent O., del Trigo M. Valve thrombosis following transcatheter aortic valve implantation: a systematic review. Revista Espanola de Cardiologia (English ed) 2015;68:198–204. doi: 10.1016/j.rec.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Latib A., Naganuma T., Abdel-Wahab M., Danenberg H., Cota L., Barbanti M. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ Cardiovasc Interv. 2015;8:e001779. doi: 10.1161/CIRCINTERVENTIONS.114.001779. [DOI] [PubMed] [Google Scholar]
- 7.Hansson N.C., Grove E.L., Andersen H.R., Leipsic J., Mathiassen O.N., Jensen J.M. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol. 2016;68:2059–2069. doi: 10.1016/j.jacc.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Leetmaa T., Hansson N.C., Leipsic J., Jensen K., Poulsen S.H., Andersen H.R. Early aortic transcatheter heart valve thrombosis: diagnostic value of contrast-enhanced multidetector computed tomography. Circ Cardiovasc Interv. 2015;8:e001596. doi: 10.1161/CIRCINTERVENTIONS.114.001596. [DOI] [PubMed] [Google Scholar]
- 9.Makkar R.R., Fontana G., Jilaihawi H., Chakravarty T., Kofoed K.F., de Backer O. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. New Engl J Med. 2015;373:2015–2024. doi: 10.1056/NEJMoa1509233. [DOI] [PubMed] [Google Scholar]
- 10.Pache G., Schoechlin S., Blanke P., Dorfs S., Jander N., Arepalli C.D. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J. 2016;37:2263–2271. doi: 10.1093/eurheartj/ehv526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography. Apical five-chamber view showing a mass in the left ventricular outflow tract.
Transesophageal echocardiography. Two-dimensional midesophageal view of the aortic valve at 120° confirmed a large burden of hypoechogenic material consistent with thrombus diffusely apposed to the ventricular side of the leaflets.
Transesophageal echocardiography. Three-dimensional midesophageal short-axis view of the prosthetic aortic valve showed severely reduced opening.
Transthoracic echocardiography at 3-month follow-up. Zoomed apical five-chamber view showing resolution of the mass in left ventricular outflow tract.