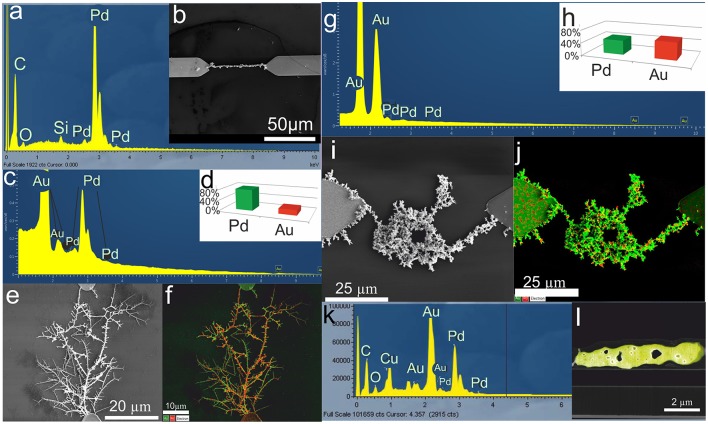
Figure 3.
(a) EDX spectrum of a Pd NW electrode, (b) SEM micrograph of the Pd NW electrode shown in (a). The Pd NW electrode was synthesized by electrodeposition from a Pd (ac)2 solution. (c) EDX spectrum of a Pd-Au nanodendrite electrode and (d) histogram of the Pd and Au element content in the Pd-Au nanodendrite shown in (e) the SEM micrograph. (f) SEM micrograph of the same Pd-Au nanodendrite electrode with elemental mapping (Pd-green, Au-red). The Pd-Au nanodendrite electrode was electrochemically assembled in a 5 × 10−3 M HAuCl4 and 5 × 10−3 M K2PdCl4 water solution at 45 MHz, 17 Vpp, and 1.5 V DC offset. (g) EDX spectrum of a Pd-Au nanodendrite electrode and (h) histogram of the Pd and Au element content in the Pd-Au nanodendrite electrode shown in (i) the SEM micrograph. (j) EDX elemental mapping of the same Pd-Au nanodendrite electrode (Pd-green, Au-red). (k) EDX spectrum of the lamella (cross section) of the Pd-Au nanodendrite electrode. (l) EDX elemental mapping of the same lamella of the Pd-Au nanodendrite electrode. The structure was synthesized by electrodeposition from 5 × 10−3 M HAuCl4 and 5 × 10−3 M PdCl2 dissolved in PBS, pH 8, at 38 MHz, 17 Vpp, and 1.5 V DC offset. Reprinted by permission from: Springer, J. Solid State Electrochemistry, Bimetallic nanowire sensors for extracellular electrochemical hydrogen peroxide detection in HL-1 cell culture, Konstantin G. Nikolaev, Vanessa Maybeck, Elmar Neumann, Sergey S. Ermakov, Yury E. Ermolenko, Andreas Offenhäusser, Yulia G. Mourzina © (2017), advance online publication, 28.11.2017 (doi: 10.1007/s10008-017-3829-3).