Figure 1.
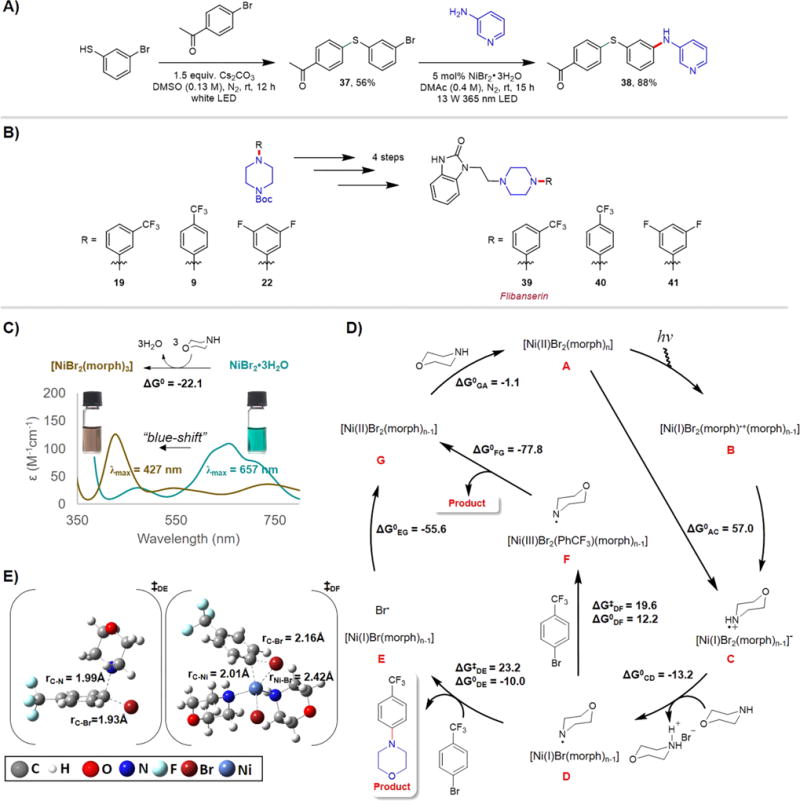
Synthetic applications and mechanistic studies. (A) Light-driven sequential C–S and C–N cross-couplings to construct molecular complexity. For the synthesis of 37, 1.0 equiv of 4′-bromoacetophenone and 1.5 equiv of 3-bromothiophenol were used; for the synthesis of 38, 1.5 equiv of 3-aminopyridine and 1.5 equiv of quinuclidine base were used. DMSO = dimethyl sulfoxide. (B) Synthesis of flibanserin and two structurally related derivatives; the yields of 19, 9, and 22 are shown in Scheme 2. Using 19, 9, and 22 as the reagents, 39, 40, and 41 were obtained in the yields of 50%, 40%, and 70%, respectively. (C) UV–vis spectra of NiBr2·3H2O and NiBr2·3H2O + morpholine in DMAc; 70 equiv of morpholine was added with respect to NiBr2·3H2O in accordance to our standard reaction conditions. Photographs showing the teal color of NiBr2·3H2O solution in DMAc transformed to brownish yellow upon morpholine addition. (D) Proposed C–N cross-coupling mechanism derived from density functional theory (DFT) calculations for n = 3. Reported free energies (in kcal/mol at 298 K and 1 M in solution) were computed at uM06/6-311+G(d,p)//uM06/6-31+G(d,p) level of theory with CPCM-described solvation in DMAc solvent. (E) Computed transition state structures for steps DE and DF (for n = 3). morph = morpholine, PhCF3Br = 4-bromobenzotrifluoride, CPCM = conductor-like polarizable continuum model; λmax is the maximum absorption wavelength and ε the molar absorptivity.
