With the completion of the human genome in 2003, it became evident what had been suspected for a time through isolated bits of data: a surprisingly small proportion of the genome – only ~2% ! – was actually translated into proteins (DNA → RNA → protein). The vast majority of the DNA had no protein correlate and was considered sterile, receiving the somewhat derisive term “junk” DNA. However, modern technology such as next-generation sequencing has revealed that almost the entire human genome is transcribed (DNA → RNA), meaning therefore that the majority of its nucleotides are associated with at least one transcript that does not code for a protein.1 What function do these non-coding RNAs (ncRNAs) serve, then? Work from several fields has painstakingly assembled an emergent picture for ncRNAs in epigenetic processes. Generally speaking, ncRNAs are main actors in the regulation of gene expression, both at the translational and post-translational level. Non-coding RNAs, which are arbitrarily divided into short ncRNAs (sncRNA, <30 nucleotides) and long ncRNAs (lncRNA, >200 nucleotides), play a critical role in heterochromatin formation, histone modification, DNA methylation and gene splicing and silencing; dysregulation of their interplay with DNA and its transcription machinery may lead to diseases like cancer, autism, and Alzheimer.2 Thus, “junk” DNA, from which ncRNAs stem from, has turned out to be anything but, revealing amazing complexity and fruitful design.
Although their expression levels are generally low, the number of known ncRNAs is already in the tens of thousands, evidently greater in magnitude than protein-coding mRNA. To be exact, a few lncRNAs have been wrongly annotated and do code for proteins, but differing from the polyvalent role of their parental lncRNAs, these proteins are known so far to circumscribe their function to modulation of other proteins. A good example is myoreguling (MLN),3 a 46-amino acid micropeptide that regulates sarcoplasmic reticulum (SR) Ca2+ levels by inhibiting SERCA1 pump activity directly. However, the protein-coding capacity of lncRNAs is an exception to the rule, and most lncRNAs control a wide range of biological processes as transcribed RNA molecules,4 by mechanisms that remain poorly understood.
In this issue of Circulation Research, Zhang et al.5 shed light on some of the molecular mechanisms underlying lncRNA regulation of biological processes. They provide evidence that Zinc-finger antisense 1 (ZFAS1), a lncRNA to the 5′ end of the protein-coding gene ZNFX1, is a major negative regulator of intracellular Ca2+ cycling in the heart, likely by repressing SERCA2a gene expression (and thus acting in a classical epigenetic process), but also via direct interaction with SERCA2a protein. A major novelty of this study, therefore, lies on unveiling the capacity of a lncRNA to regulate a target protein directly, via RNA-protein interactions. Although the case of ZFAS1 direct interaction with SERCA2a was inferred by Zhang et al.5 and may require further experimental support, this mechanism if confirmed would add to the broad repertoire of strategies that lncRNAs use to modulate biological functions.
In addition to the novel concepts that Zhang et al.5 bring to the broader field of lncRNAs, their study uncovers a major role for ZFAS1 on cardiac myocyte Ca2+ handling and its dramatic effect on myocardial infarction (MI). In a previous study by the same group,6 ZFAS1 levels in blood were found decreased in humans post-MI, and a mouse model of MI confirmed this finding, opening the possibility of using ZFAS1 as a biomarker of MI. In addition, ZFAS1 levels were increased in MI tissue,6 and this observation led to the eternal dilemma that pervades most studies that find a molecule altered in disease, namely, is the molecule causal in the pathogenesis of the disease, or a simple bystander? Zhang et al.5 addressed here the relationship of ZFAS1 to MI with elegant experiments. They found that MI induced a significant increase in the basal cytoplasmic and SR levels of ZFAS1, and that knocking down endogenous ZFAS1 lessened the contractile dysfunction brought about by MI. Conversely, artificially overexpressing ZFAS1 in healthy mice recapitulated some of the cardiac dysfunction observed in MI mice. At the cardiomyocyte level, the deleterious effects of ZFAS1 overexpression were observed as altered Ca2+ transients and intracellular Ca2+ overload, which likely contributed to the weakened contractility of whole hearts. The capacity of ZFAS1 to elicit cellular, whole heart and intact animal phenomena characteristic of MI was persuasive evidence of its hierarchical importance in the cascade of events triggered by this pathological state. By Koch’s postulates, ZFAS1 appears to be a causal agent in the pathogenesis of MI, not its byproduct.
Zhang et al.5 also uncovered at least some of the molecular mechanisms underlying ZFAS1 detrimental effect on cardiac contractility. Apparently, a major role of ZFAS1 is to limit Ca2+ uptake by the SR, which it exerts by a) using a discrete functional domain (ZFAS1-FD) conserved among species to bind to SERCA2a protein and inhibit its activity, and b) repressing SERCA2a-encoding ATP2A2 gene expression. This dual-prong approach disrupts Ca2+ homeostasis and leads to intracellular Ca2+ overload, in turn negatively affecting myofilament proteins, gene expression, Ca2+ entry and extrusion, and presumably many other processes controlled by Ca2+-signaling mechanisms. Zhang et al.,5 however, do not hypothesize on a potential mechanism that may instruct cardiomyocytes to increase ZFAS1 levels after the ischemic insult. All they find is that NFATc2 is activated after MI, which then increases ZFAS1 expression (Fig. 1). In a recent study, Wu et al.7 found that ZFAS1 promotes cardiomyocyte apoptosis by acting as a competing endogenous RNA to miR-150 and increasing C-reactive protein expression. Given the wide spectrum of processes used by lncRNAs, it would not be surprising that ZFAS1 may affect cardiac function by modulating intracellular Ca2+ and apoptotic cell death, two different but potentially interdependent pathways.
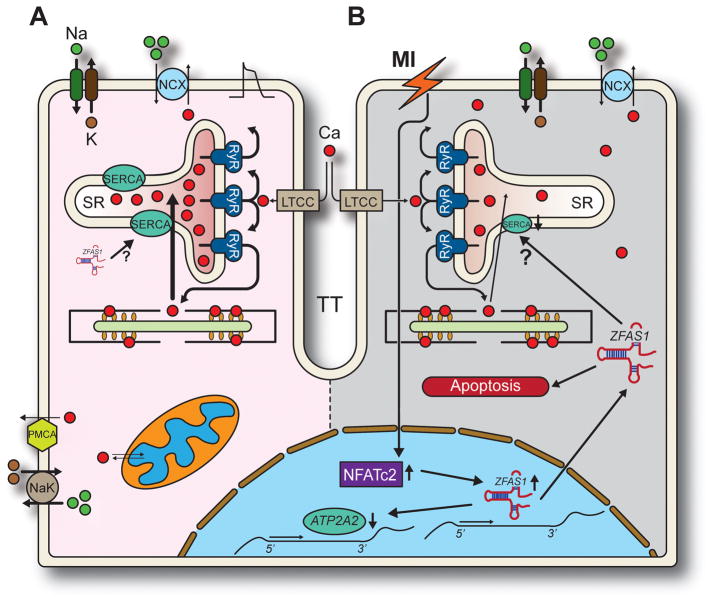
Figure 1.
Proposed mechanisms underlying ZFAS1 modulation of cardiac function in normal and MI-induced states. A: Normal excitation-contraction coupling. ZFAS1 is expressed at low levels and while it may interact with SERCA, excitation-contraction coupling proceeds typically: action potentials trigger an inward Ca2+ current through L-type Ca2+ channels (LTCC), activating Ryanodine Receptors (RyR). RyRs release Ca2+ from the SR producing contraction of the myofilaments. Relaxation occurs when SERCA2a recaptures Ca2+ into the SR and NCX extrudes it from the cell. PMCA: plasma membrane Ca2+ ATPase; NaK: Na+/K+ pump. B. The ischemia and tissue hypoxia produced by MI promotes upregulation of ZFAS1 through unknown mechanisms that involve NFATc2. ZFAS1 then decreases expression of SERCA2a-encoding ATP2A2 gene. While ZFAS1 also interacts with SERCA2a, it is unclear whether this entails direct regulation of pump activity (?). Ultimately, reduced SERCA2a expression and function contributes to lower SR Ca2+ content, increases cytosolic Ca2+ and ends in contractile dysfunction. Increased ZFAS1 also induces apoptosis through yet unidentified mechanisms,7 which may also contribute to overall cardiac dysfunction following MI.
Zhang et al.5 derive from their work some important implications for our consideration. First, they tout ZFAS1 as independent predictor of acute MI, but is it realistic to use ZFAS1 as a biomarker of disease severity in MI and perhaps other cardiac diseases, or as prognostic tool or risk factor? This is so far a hopeful wish, given that existent biomarkers for MI such as creatine kinase MB (CKMB) and cardiac Troponin I (cTnI) perform sub-optimally in the clinical arena;8 nonetheless, the fact that blood levels of ZFAS1 decrease significantly after MI,6 makes it a tantalizing possibility. Second, Zhang et al.5 observed that downregulation of ZFAS1 levels in heart not only prevented but also reversed major deleterious consequences of MI, thus advancing the notion that anti-ZFAS1 maneuvers may be effective therapies for certain cardiomyopathies. But their major ground for this rationale is their finding that inhibiting ZFAS1 in the heart improves SERCA2a function, a template that has failed to benefit heart failure patients in a large-scale clinical trial.9 If history serves any purpose here, it might therefore appear speculative to propose that a complex condition such as MI, where many and apparently independent cellular processes and pathways converge in dramatically deranged functional and structural end-points, may be stopped or even reversed by improving the activity of a single molecule (SERCA2a). Soberly, Zhang et al.5 acknowledge limitations by pointing to the fact that SERCA2a depression was lower than cardiac deterioration after MI, implying additional intervening factors in ZFAS1 pathway to trigger overt cardiac dysfunction. Again, this would be of little surprise considering the broad array of biological processes affected by lncRNAs in general2 and ZFAS1 in particular.10 ZFAS1 has been previously cast as a foe due to its implication in several human cancers,10 but if evolutionary pressure has not weed it out yet from our genome is because it must serve a desirable function, meaning that is also a friend. Indeed, suppression of ZFAS1 expression appears to promote cell proliferation in breast cancer.11 The challenge, therefore, for cardiac and cancer therapies, will be to find the right balance between suppression and preservation of ZFAS1 function, avoiding its undesirable activity while maintaining its most indispensable services.
The increasing evidence for the role of lncRNAs in pathophysiological processes cast them as potential therapeutic targets, not only in cardiac disease but beyond. Still, significant hurdles lie ahead as the field of lncRNAs, as vast as it is, is still in infancy and holds many unknowns.
Acknowledgments
Sources of Funding: Supported by National Institutes of Health grants R01-HL055438, R01-HL120108 and R01-HL134344 (to HHV).
Footnotes
Disclosures: None
References
- 1.ENCODE Project Consortium. Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maass PG, Luft FC, Bahring S. Long non-coding RNA in health and disease. J Mol Med (Berl) 2014;92:337–346. doi: 10.1007/s00109-014-1131-8. [DOI] [PubMed] [Google Scholar]
- 3.Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, Cannon SC, Houser SR, Bassel-Duby R, Olson EN. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351(6270):271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon RA, Jaé N, Holdt L, Dimmeler S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J Am Coll Cardiol. 2016;67(10):1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Jiao L, Sun LH, Li Y, Gao Y, Xu C, Shao Y, Li M, Li C, Lu Y, Pan Z, Xuan LN, Zhang Y, Li Q, Yang R, Zhuang Y, Zhang Y, Yang B. LncRNA ZFAS1 as a SERCA2a Inhibitor to Cause Intracellular Ca2+ Overload and Contractile Dysfunction in a Mouse Model of Myocardial Infarction. Circ Res. 2018 Feb 23; doi: 10.1161/CIRCRESAHA.117.312117. pii: CIRCRESAHA.117.312117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Sun L, Xuan L, Pan Z, Li K, Liu S, Huang Y, Zhao X, Huang L, Wang Z, Hou Y, Li J, Tian Y, Yu J, Han H, Liu Y, Gao F, Zhang Y, Wang S, Du Z, Lu Y, Yang B. Reciprocal changes of circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute myocardial infarction. Sci Rep. 2016;6:22384. doi: 10.1038/srep22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Wu D, Wu Q, Zou B, Huang X, Cheng X, Wu Y, Hong K, Li P, Yang R, Li Y, Cheng Y. Knockdown of Long Non-Coding RNA-ZFAS1 Protects Cardiomyocytes Against Acute Myocardial Infarction Via Anti-Apoptosis by Regulating miR-150/CRP. J Cell Biochem. 2017;118(10):3281–3289. doi: 10.1002/jcb.25979. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48:1–11. doi: 10.1016/j.jacc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 9.Hulot JS, Salem JE, Redheuil A, Collet JP, Varnous S, Jourdain P, Logeart D, Gandjbakhch E, Bernard C, Hatem SN, Isnard R, Cluzel P, Le Feuvre C, Leprince P, Hammoudi N, Lemoine FM, Klatzmann D, Vicaut E, Komajda M, Montalescot G, Lompré AM, Hajjar RJ AGENT-HF Investigators. Effect of intracoronary administration of AAV1/SERCA2a on ventricular remodelling in patients with advanced systolic heart failure: results from the AGENT-HF randomized phase 2 trial. Eur J Heart Fail. 2017;19(11):1534–1541. doi: 10.1002/ejhf.826. [DOI] [PubMed] [Google Scholar]
- 10.Lan T, Lan X, Li G, Zheng Z, Zhang M, Qin F. Prognostic role of long noncoding RNA ZFAS1 in cancer patients: a systematic review and meta-analysis. Oncotarget. 2017;8(59):100490–100498. doi: 10.18632/oncotarget.19162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan S, Fan C, Liu N, Huang K, Fang X, Wang K. Downregulation of the long non-coding RNA ZFAS1 is associated with cell proliferation, migration and invasion in breast cancer. Mol Med Rep. 2018 Mar 9; doi: 10.3892/mmr.2018.8707. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]