Abstract
Field and laboratory studies have shown that mayflies (Ephemeroptera) tend to be relatively sensitive to elevated major ion concentrations, but little is known about how ionic composition influences these responses. This study evaluated the acute toxicity of major ion salts to the mayfly Neocloeon triangulifer over a range of background water quality conditions. The mayfly was particularly sensitive to Na2SO4, with the median lethal concentration (LC50) of 1,338 mg SO4/L being lower than LC50s reported for seven other species at that hardness. Increasing hardness of the dilution water from 30 to 150 mg/L (as CaCO3) resulted in doubling of LC50s for sodium salts, and a ~1.5 fold increase in LC50 for MgSO4. Potassium salt toxicity was not strongly influenced by hardness, consistent with findings for other species. When hardness was held constant, but Ca:Mg ratio was manipulated, the ameliorative effect on Na2SO4 and NaCl did not appear as strong as when hardness was varied, but for MgSO4 the amelioration relative to Ca activity was similar between the two experiments. The toxicity of K salts to N. triangulifer was similar to Na salts on a mM basis, which contrasts with several other species for which K salts have been found much more toxic. In addition, the toxicity of KCl to N. triangulifer was not notably affected by Na concentration, as has been shown for Ceriodaphnia dubia. Finally, plotting LC50s in terms of ion activity (Cl, SO4, Na, Mg, or K) over the range of Ca activities in dilution water resulted in significant positive relationships, with comparable slopes to those previously observed for C. dubia over the same range of Ca activities.
Keywords: Neocloeon triangulifer, mayfly, acute toxicity, major ions, salinity
INTRODUCTION
The environmental impacts of elevated concentrations of major ions (i.e., Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−) have received considerable research attention in the two decades since it was recognized that major ion toxicity varies widely depending on composition of the solution [1]. The primary sources of elevated major ions in freshwaters were reviewed by Goodfellow et al. [2], but much of the recent North American literature has focused on issues related to road salting and mining [3,4], with toxicity testing primarily focused on sodium chloride or sodium sulfate tested with standard test organisms, especially crustaceans [5–13].
A number of field studies have shown that mayflies (Ephemeroptera) appear to be more sensitive than other benthic macroinvertebrate taxa to elevated major ion concentrations in streams impacted by mining [14–18], and several recent laboratory studies have generated chronic toxicity data confirming this sensitivity [19–24; J. Jackson, Stroud Water Research Center (SWRC) personal communication]. Until relatively recently, difficulties with culturing have precluded the use of mayflies as laboratory toxicity testing organisms to any great extent; however, researchers at SWRC developed a method for testing a parthenogenetic species (Neocloeon triangulifer) that readily reproduces under laboratory conditions [25]. Furthermore, recent efforts to develop laboratory cultured diets and chronic toxicity testing methods for mayflies have been successful, and will help to move toward standardization of methods for this species [23, 24, 26]. In acute (96-h) exposures reported by Soucek and Dickinson [23], sodium chloride median lethal concentrations (LC50s) for N. triangulifer were approximately equal to those reported for the cladoceran Ceriodaphnia dubia [1], but sodium sulfate LC50s were about half those for C. dubia. In a full-life chronic toxicity test, the percent emergence (the most sensitive endpoint in that test) 20% effect concentration (EC20) for N. triangulifer exposed to sodium sulfate [23], was 15 to 23% of the lowest values reported for other invertebrates including Ceriodaphnia dubia, Lampsilis abrupta, and Chironomus dilutus [13]. The most sensitive chronic response level of N. triangulifer to sodium chloride [23] was 36 to 39% of those of C. dubia and Daphnia magna [6] and similar to the response of the chloride sensitive mussel Lampsilis siliquoidea (N. Wang et al. 2015, poster at SETAC North America annual conference, Salt Lake City, UT).
The fact that major ion toxicity to standard laboratory test organisms like cladocerans, amphipods, and fish is dependent on the ionic composition of a water or effluent has been well established; for example, several studies have shown that the toxicity of sodium sulfate and sodium chloride decrease with increasing water hardness [1, 5–7, 9–12], and more specifically, increasing calcium concentration [5, 9]. It is also known that for crustaceans and fish, solutions tend to be more toxic when dominated by particular major ions; C. dubia is more sensitive to solutions dominated by K+, Mg+, and HCO3− than those dominated by Na+, Cl−, and SO42− [1, 9]. Furthermore, recent work has shown that sodium concentration regulates the toxicity of potassium salts to C. dubia [9]. Very little is known about how ionic composition influences responses of N. triangulifer to elevated major ions, although Kunz et al. [22] observed differences in chronic responses of this species to Na+ and SO42− dominated solutions compared to Ca2+, Mg2+, SO42−, and HCO3− dominated solutions, and SWRC (J. Jackson, personal communication) noted differences in 48-h NaCl LC50s in two dilution waters with different hardnesses. Zalizniak et al. [27] investigated the influence of ionic composition on salinity toxicity to the related Australian mayfly Centroptilum sp., but test concentrations were not sufficiently low to have confidence in LC50s. Because N. triangulifer appears to be more sensitive to some major ions than other laboratory cultured test organisms, greater knowledge of the influence of dilution water composition on major ion toxicity will be important to refining our ability to evaluate potential risks to aquatic communities.
While most field studies [e.g., 14–16] and some laboratory or mesocosm studies [19, 20, 22, 28, 29] report responses to elevated major ions in terms of electrical conductivity, endpoints for major ion laboratory toxicity tests are most frequently reported in terms of anion concentrations [e.g., 5–8, 10–13]. The focus on anions may be due in part to the finding of Mount et al. [1] that Na and Ca were not significant factors in regressions used to model ion toxicity; however, more recent studies by Erickson et al. [30] suggested K, Mg, and Ca salt toxicities to C. dubia are related to the cations, and that Na salt toxicity may be related to multiple ions or osmolarity (i.e., not necessarily the associated anion). These findings with C. dubia indicate the need to re-examine drivers of acute effects due to various major ion salts. In the present study we report acute toxicity of various major ion salts in terms of both anion and cation concentrations, but a subsequent paper will be devoted to more explicitly evaluating likely causes of toxicity to this species in major ion salt toxicity tests.
The primary goal of the present study was to determine how changes in ionic composition of dilution water impact responses of N. triangulifer to elevated major ions over a range of background water quality conditions. Building on the work of Mount et al. [9] with C. dubia, we investigated how changes in hardness, and more specifically, changes in calcium impact acute toxicity of several major ion salts. We also tested the reciprocal influences of Na on KCl toxicity and K on NaCl toxicity.
METHODS
Culturing of test organisms and food
The parthenogenetic mayfly, Neocloeon triangulifer (family Baetidae) [31] was originally described as Cloeon triangulifer, later transferred to the genus Centroptilum [32] and most recently assigned to Neocloeon [33]. The genetic strain we used was Stroud Water Research Center Clone #WCC-2™. It is reared in the laboratory on a diatom diet, and mayfly and diatom biofilm diet culturing methods were similar to those reported in Soucek and Dickinson [23]. Diatoms used to feed mayflies included Mayamaea sp., and Nitzschia sp. Both diatoms were obtained from Carolina Biological Supply Co. (Burlington, NC), sold as Navicula sp. and Synedra sp., respectively. We had the genus level identities taxonomically confirmed by an expert (S. Decelles) at USEPA-Office of Research and Development, Cincinnati, OH.
Mixed diatom stocks
To culture diatoms, we autoclaved (30 min. at 121 °C, liquid cycle) a 4-L flask containing 4 L of filtered (Whatman™ 934-AH) dechlorinated tap water and a 2-inch long, Teflon® coated stir bar. After allowing to cool, sterile technique was used to add 1.3 ml of Kent® – Proculture Professional F/2 Algal culture formula A, 1.3 ml of Kent® – Proculture Professional F/2 Algal culture formula B, 150 mg of sodium metasilicate (Na2SiO3·9H2O), and 200 ml of fresh diatom stock solution (just removed from stir-plate). Both diatom species were present in combination in stock cultures. The flasks were placed on stir-plates with moderate to fast stirring (a large vortex was visible) in an environmental chamber set for a 16 h:8 h (L:D) photoperiod and 25 °C. Light intensity in the chamber varied between 800 and 1200 lux depending on position in the chamber. Diatom stocks were allowed to grow for five days, then 200 ml of stock was used to seed the next flask and cages for mixed diatom slides (see below). Allowing growth for much more than five days appeared to cause depletion of nutrients and cell death. Stocks were not refrigerated prior to seeding subsequent flasks or mixed diatom slide cages.
Mixed diatom slides
To culture mixed diatom slides, fifteen fully frosted microscope slides (Cat. No. 12-544-5CY, www.fishersci.com) were placed in a single layer (with frosted side facing up) on the bottom of a 7.2-L (189 mm × 297 mm × 128 mm) autoclavable polysulfone mouse cage (#PC7115HT, www.acecaging.com) filled with 2.5 L of filtered (Whatman™ 934-AH) dechlorinated (carbon-filtered and aged) tap water. The container with the slides was autoclaved (30 min. at 121 °C, liquid cycle) and allowed to cool. Sterile technique was used to add 1.3 ml of Kent® – Proculture Professional F/2 Algal culture formula A, 1.3 ml of Kent® – Proculture Professional F/2 Algal culture formula B, 150 mg of sodium metasilicate (Na2SiO3·9H2O; dissolved in a small amount of deionized water prior to addition), and 200 ml of fresh (never refrigerated) mixed diatom stock. The container with slides was covered with clear plastic wrap and placed in an environmental chamber set for a 16 h:8 h (L:D) photoperiod and 25 °C. Light intensity at the level of the slides varied between 300 and 100 lux, depending on position in the chamber. Growth was allowed for 6 to 10 days. Soucek and Dickinson [23] provided further details on assessing biofilm quality prior to feeding to mayflies.
Mayfly nymph rearing method
Mayflies were reared in an environmental chamber at 25 °C, a photoperiod of 16 h:8 h (L:D) and light intensity of ~200 lux. Culture water was a reconstituted water (hereafter referred to as Duluth 100) with a nominal hardness of 100 mg/L as CaCO3, prepared according to a formula developed at the U.S. EPA laboratory in Duluth, MN (Table S1, S2). This water recipe was designed with the goal of better mimicking chemistry of “typical” North American freshwaters relative to other commonly used reconstituted waters. When eggs hatched, ~250 ml of culture water were added to a 300 ml clear glass jar. All water was filtered using Whatman™ #934-AH glass microfiber filters. One mixed diatom slide was added to the jar. Newly hatched mayfly larvae (100s to 1000s) were then added to the jar, the lid was loosely replaced, and the jar was covered with aluminum foil to block direct overhead lighting. When mayflies were 4 to 8 days old (usually 6 or 7 days), 40 individuals were placed in a 1-L glass beaker containing 400 ml Duluth 100 reconstituted water, and fed as described for the 300-ml glass jar. The diatom slide was placed in the beaker prior to adding mayflies to avoid injury. Again, the container was covered with aluminum foil to block direct overhead lighting. When mayflies were 11 to 12 days old, 20 individuals were transferred to a 19 × 24 × 6.5 cm Pyrex casserole dish containing 1.5 L of Duluth 100 water and five mixed diatom slides. Slides were replaced when diatom biofilms were depleted, and water was changed twice per week or more if water appeared to be littered with loose diatoms and waste products. The container was covered loosely with foil. Using this method, aeration was not necessary at any point during mayfly culturing.
When pre-emergent nymph stages (PEN, determined by presence of black wing pads) appeared (days 20 – 23), they were placed in a 300 ml I-chem jar containing culture water and a mixed diatom slide. A screened cover was placed on the jar to allow for emergence of sub-imagoes and molting to imago stage (within 24 h after PEN stage). To induce the imago to release its eggs, we held it by the wings with forceps and touched its abdomen to water held in a small petri dish. This procedure was conducted with the aid of a dissecting microscope. Eggs were then pipetted into a scintillation vial; when possible, eggs of 3 females were combined in each vial. Eggs were either allowed to hatch or placed in an environmental chamber at 10 °C for later use.
General acute toxicity testing procedures
Static, non-renewal, acute toxicity tests were conducted according to guidelines detailed in ASTM E729-96 [34]. Five concentrations spaced by 50% dilution were tested in addition to controls. Further details on test conditions are provided in Table S3. Eggs were transferred to test water prior to hatching so that organisms hatched into the water in which they would be tested, and organisms were <24-h-old at the beginning of a test. Test chambers were fed by grasping a mixed diatom slide (cultured as described above) with a forceps, and scraping off a ~5- to 10- by 25-mm area of biofilm with another clean microscope slide, and releasing the biofilm into each replicate test chamber. Test organisms were fed because in a previous study first instar mayflies had 22% survival after 48 h with no food [23]. This has been observed by others as well [24; J. Jackson, SWRC, personal communication]. Because all tests were conducted with major ions, food was not expected to impact dissolved concentrations of the contaminants, and analytical chemistry confirmed this (detailed below). Food was added to test chambers only on day zero as previous testing demonstrated one biofilm scraping was more than enough for the 96-h test duration. Mortality was assessed daily using a dissecting microscope. Individuals were considered dead if they did not respond to gentle prodding with a probe.
Temperature, pH, conductivity, and dissolved oxygen were measured in all treatments at the beginning and the end of each exposure period. Alkalinity and hardness were measured in the controls and the highest test treatment at the beginning of the test only. The pH measurements were made using an Accumet® (Fisher Scientific, Pittsburgh, PA, USA) model AB15 pH meter equipped with an Accumet® gel-filled combination electrode (accuracy < ± 0.05 pH at 25 °C). Dissolved oxygen was measured using an air-calibrated Yellow Springs Instruments (RDP, Dayton, OH, USA) model 55 meter. Conductivity measurements were made using a Mettler Toledo® (Fisher Scientific, Pittsburgh, PA, USA) model MC226 conductivity/TDS meter. Alkalinity and hardness were measured by titration as described by American Public Health Association [35]. Water samples from each treatment were collected at the beginning and end of tests and submitted to the IL State Water Survey analytical laboratory for verification of selected major ion concentrations using ion chromatography.
Experimental approach and dilution waters
We developed a testing approach that covered three basic objectives which are detailed in this section. Recipes for and detailed chemistry of the nine different dilution waters used are provided in Tables S1 and S2. Reagent grade or certified ACS grade salts were used to make all test waters. Solutions enriched with MgSO4 were prepared using MgSO4*7H2O or MgSO4 (anhydrous), but are reported as MgSO4.
Hardness comparisons
The first set of tests determined the toxicity NaCl, Na2SO4, K2SO4, and MgSO4 in dilution waters representing different hardnesses. Waters with nominal hardnesses of 30, 90, 150, and 210 mg/L (as CaCO3) were prepared using recipes developed at the U.S. EPA laboratory in Duluth, MN, which varied all major ions to approximate average concentrations in U.S. surface waters of a given hardness (Tables S1, S2). These test waters differed from culture water (Duluth 100) in that CaCO3 was the main source of carbonate rather than NaHCO3. Carbon dioxide gas (99.9% CO2) was bubbled through the solution (to ~pH 5.2) to dissolve CaCO3, then natural air was bubbled through the solution to bring the pH back to ~7.6 for testing.
Ca:Mg comparisons
While hardness tests evaluated the aggregate effect of varying all ions in dilution water, additional tests varying Ca:Mg ratios at constant hardness were used to evaluate the specific role of calcium. Toxicity of NaCl, Na2SO4, K2SO4, and MgSO4 were tested in waters similar to Duluth 100 water but modified to “low Ca:Mg” (0.5 ratio on a mass basis), and “high Ca:Mg” (5.0 mass ratio) compositions with all other ions held constant (Tables S1, S2).
Na/K interactions
The reciprocal influences of Na on KCl toxicity and K on NaCl toxicity were tested by comparing responses in Duluth 100 water (which served as a low Na or low K water) to responses in Duluth 100 water with high Na (150.3 mg Na/L added as NaCl) or high K (15.0 mg K/L added as KCl see Table S2). These comparisons were spurred by the finding of Na-dependent toxicity of KCl in C. dubia, [9] and K-dependent toxicity of Na2SO4 in fathead minnows [13].
Data analysis
For each acute toxicity test, the dominant anion concentrations were analytically verified in each treatment as described above. We calculated median lethal concentrations (LC50s) for each test in terms of measured dominant anion concentration (mg/L) using the trimmed Spearman-Karber method [36]. Then, based on measured anion concentration, calculated concentration of the associated cation (mg/L), and nominal concentrations of other ions in the dilution water, we estimated all major ion concentrations (mM) at the LC50 value for each test (Table 1 and Table S4). We then used Visual MINTEQ, version 3.0 (JP Gustafsson, Royal Institute of Technology, Department of Land and Water Resources Engineering, Stockholm, Sweden) to calculate the activity of all potential ionic species at the LC50 concentration for each test (Table S5). For activity modelling, we input fixed pH values based on the average measured pH for each test. Calcium was the most frequently manipulated major ion in dilution waters in this study, and previous studies with Ceriodaphnia dubia indicated that calcium influenced toxicity of Na and Mg salts [9]. Therefore, to evaluate the influence of Ca on major ion toxicity, we created scatter plots with LC50s in terms of Cl, SO4, Na, Mg, or K activities as dependent variables, and Ca activity as the independent variable. For each dependent variable ion, we included all salts tested, for example, for SO4 LC50s, we included K2SO4, MgSO4, and Na2SO4. We also plotted potassium salt LC50s against sodium activity as the independent variable because sodium regulated potassium salt toxicity to C. dubia [9].
Table 1.
96-h median lethal concentrations for Neocloeon triangulifer for single major ions salts in various dilution waters.
| Hardness comparisons | ||||
|
| ||||
| Salt | Dilution water | measured hardness mg/L as CaCO3 | LC50 (95% C.I.) mg anion/L | LC50 1 mg cation/L |
|
| ||||
| NaCl | Recon 30 | 30 | 490 (424 – 566) | 319 |
| NaCl | Recon 90 | 88 | 837 (706 – 993) | 545 |
| NaCl | Recon 150 | 141 | 1128 (1059 – 1201) | 735 |
| NaCl | Recon 210 | 205 | 1116 (929 – 1341) | 732 |
| Na2SO4 | Recon 30 | 31 | 728 (614 – 863) | 349 |
| Na2SO4 | Recon 90 | 88 | 1338 (1166 – 1535) | 639 |
| Na2SO4 | Recon 150 | 142 | 1758 (1511 – 2045) | 837 |
| Na2SO4 | Recon 210 | 210 | 1822 (1583 – 2098) | 865 |
| K2SO4 | Recon 30 | 30 | 1017 (887 – 1166) | 821 |
| K2SO4 | Recon 90 | 87 | 1070 (918 – 1248) | 854 |
| K2SO4 | Recon 150 | 141 | 1024 (857 – 1224) | 803 |
| K2SO4 | Recon 210 | 205 | 1808 (1580 – 2069) | 1418 |
| K2SO4 | Recon 210 | 212 | 1261 (1107 – 1437) | 973 |
| MgSO4 | Recon 30 | 28 | 1348 (1231 – 1478) | 341 |
| MgSO4 | Recon 90 | 86 | 1621 (unreliable) | 411 |
| MgSO4 | Recon 150 | 140 | 2112 (1814 – 2460) | 536 |
| MgSO4 | Recon 210 | 212 | 1836 (1571 – 2145) | 463 |
|
| ||||
| Ca:Mg comparisons | ||||
|
| ||||
| Salt | Dilution water | measured hardness mg/L as CaCO3 | LC50 (95% C.I.) mg anion/L | LC50 mg cation/L |
|
| ||||
| NaCl | low Ca:Mg | 95 | 905 (728 – 1125) | 603 |
| NaCl | high Ca:Mg | 93 | 1086 (913 – 1293) | 721 |
| Na2SO4 | low Ca:Mg | 94 | 1229 (1022 – 1477) | 595 |
| Na2SO4 | high Ca:Mg | 93 | 1427 (1211 – 1682) | 689 |
| K2SO4 | low Ca:Mg | 95 | 1017 (789 – 1312) | 785 |
| K2SO4 | high Ca:Mg | 93 | 1164 (1062 – 1275) | 904 |
| MgSO4 | low Ca:Mg | 95 | 872 (758 – 1002) | 224 |
| MgSO4 | high Ca:Mg | 94 | 1436 (1152 – 1790) | 355 |
|
| ||||
| Na/K interactions | ||||
|
| ||||
| Salt | Dilution water | measured hardness mg/L as CaCO3 | LC50 (95% C.I.) mg anion/L | LC50 mg cation/L |
|
| ||||
| KCl | low Na (Duluth 100) | 93 | 1226 (1043 – 1441) | 1326 |
| KCl | high Na | 92 | 1221 (984 – 1516) | 1123 |
| NaCl | low K (Duluth 100) | 92 | 910 (719 – 1153) | 606 |
| NaCl | low K (Duluth 100) | 94 | 1153 (968 – 1374) | 764 |
| NaCl | high K | 91 | 1012 (834 – 1228) | 666 |
Dilution water composition was considered in estimating cation concentration at LC50
QA/QC
Control survival was 100% in the majority of the acute toxicity tests reported here, and always ≥90%.
Across all acute toxicity tests conducted, the mean (± standard deviation) overall temperature, pH, and dissolved oxygen values were 25.0 ± 0.4 °C, 8.2 ± 0.3 standard units, and 7.7 ± 1.3 mg/L, respectively. The lowest dissolved oxygen concentration observed in any test was 6.4 mg/L. Specific conductivity, alkalinity, and hardness varied depending on dilution water and test treatment, but were consistent with the nominal composition of the treatment.
For the acute toxicity tests with chloride salts, measured Cl− concentrations averaged 104% of nominal (range = 92 to 118%). For the acute toxicity tests with sulfate salts, measured SO42− concentrations averaged 103% of nominal (range = 87 to 124%). Measured concentrations were used for calculating LC50s, and reported cation concentrations were calculated proportionally based on measured concentrations of anions.
RESULTS AND DISCUSSION
Hardness comparisons
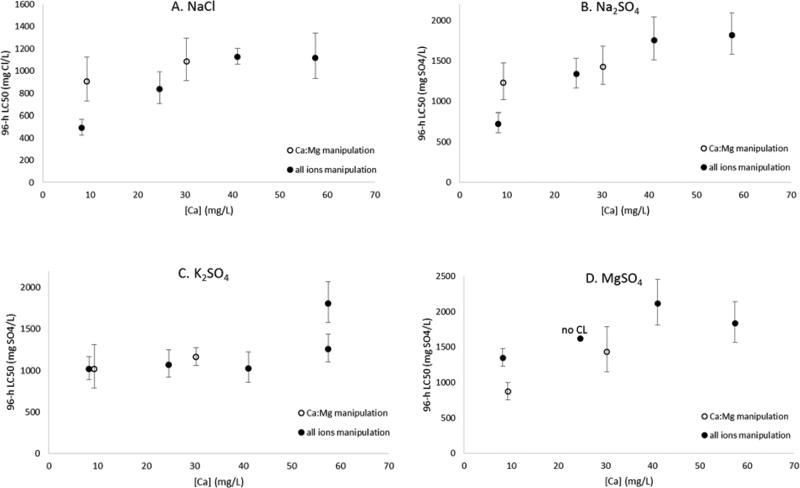
When tested across dilution waters with different hardness, NaCl, Na2SO4, and MgSO4 all decreased in toxicity with increasing hardness, though to varying degrees (Table 1, Fig. 1). For both NaCl and Na2SO4, the LC50 more than doubled as hardness increased from about 30 to 150 mg/L (nominal hardnesses), whereas for MgSO4 the increase was approximately 1.5 fold. Despite this rise, all three of these salts showed little difference in toxicity between 150 and 210 mg/L hardness. For K2SO4, the pattern was markedly different, with little difference among the 30, 90, and 150 mg/L waters. For the 210 hardness water, the initial test resulted in an LC50 of 1,808 mg Cl/L, roughly 1.8× above that in the lower three hardnesses. As this pattern was unexpected, the test at 210 mg/L hardness was repeated, yielding an LC50 of 1,261 mg Cl/L, much closer to the lower three (Table 1). Therefore, it is unclear whether K2SO4 toxicity is hardness dependent, but it appears to be less so than the other three salts.
Figure 1.
Influence of calcium (Ca) in dilution water on the acute toxicity of A.) NaCl, B.) Na2SO4, C.) K2SO4, and D.) MgSO4 to the mayfly Neocloeon triangulifer. “All ions manipulation” are data from the “hardness comparisons” series of tests in the present study; Ca concentrations of 8, 25, 41, and 58 mg/L were waters with 30, 90, 150, and 210 mg/L as CaCO3 nominal hardness, respectively. Error bars are 95% confidence limits (CL).
Hardness dependent toxicities of NaCl and Na2SO4 have been reported for several other species previously. For NaCl, C. dubia [9, 12], the snail Gyraulus parvus [12], the unionid mussel L. siliquoidea [37], and the worm Tubifex tubifex [12] have been observed to exhibit hardness-dependent acute sensitivity. In addition, SWRC (J. Jackson, personal communication) documented decreased NaCl toxicity to the mayflies N. triangulifer, Procloeon rivulare, and Pseudocloeon frondale in 48-h toxicity tests using a natural water with a hardness of 105 mg/L relative to a natural water with a hardness of 22 mg/L. Likewise for Na2SO4, hardness dependent sensitivity has been observed for C. dubia [9, 10], Daphnia magna [5], and H. azteca [5, 11].
Ca:Mg comparisons
In the dilution waters used for hardness comparisons, all of the major ions increased with increasing hardness, not just Ca and Mg (Table S2). This was done purposefully to examine trends in waters that simulate how toxicity might vary in natural waters for which concentrations of all major ions generally co-vary with increasing hardness. Research on other organisms has suggested that the ameliorative effect of hardness is largely due to increased Ca specifically [5, 9]; to evaluate this relationship for N. triangulifer, we tested toxicity of the same four salts (NaCl, Na2SO4, K2SO4, and MgSO4) in dilution waters with constant nominal hardness but different Ca:Mg ratios. Both waters had measured hardnesses of 93 to 95, but the “low Ca:Mg” water had a calcium concentration similar to that in the “Recon 30” water (nominally 9.3 mg Ca/L) while Ca in the “high Ca:Mg” was more than three times higher (30.3 mg Ca/L), slightly higher than that in “Recon 90” (Table S2). If the variations in LC50s in the “hardness comparisons” series were attributable only to calcium, we would expect shifts in LC50 from the Ca:Mg manipulation paralleling those in the hardness comparison studies.
In the case of the two sodium salts, the actual differences in LC50 from low Ca:Mg to high Ca:Mg were less than predicted by the “hardness comparison” tests (Table 1; Fig 1). The fact that their LC50s increased less when manipulating only Ca:Mg rather than all ions suggests either that something aside from or in addition to calcium accounted for the decreased toxicity in the “hardness comparisons” series, or that the higher overall ion concentrations in the low Ca:Mg water relative to 30 hardness water provided some benefit (Table S2). Conversely, the MgSO4 results showed a similar increase in LC50 when only calcium was increased, and when all ions increased proportionally as in the hardness comparison (Table 1; Fig 1); this suggests that calcium accounted for most or all of the modification of toxicity due to hardness. The K2SO4 results were consistent with the previous tests in the hardness series, showing little, if any effect of Ca or hardness on toxicity over the range present in the lower three hardness waters (Table 1; Fig 1). The hardness/Ca dependence of toxicity for Na and Mg salts but not K salts, shown here for N. triangulifer, is consistent with the findings of Mount et al. [9] for C. dubia.
Na/K interactions
In the Na/K interactions series, we observed essentially no effect on KCl toxicity when Na varied from 34 mg/L (“low Na” = Duluth 100; Table S2) to 150 mg/L (high Na; Table S1, S2), with 96-h LC50s of 1,226 and 1,221 mg Cl/L, respectively (Table 1). Likewise, the mean LC50 for the two NaCl tests conducted at “low K” (3.9 mg/L) was 1,032 mg Cl/L, whereas the LC50 at “high K” (15 mg/L) was 1,012 mg Cl/L (Table 1). The lack of influence of Na on the toxicity of KCl contrasts with the marked ameliorative effect of Na on KCl toxicity reported for C. dubia [9], while the lack of influence of K on toxicity of NaCl is parallel with that found for C. dubia.
Ion activities
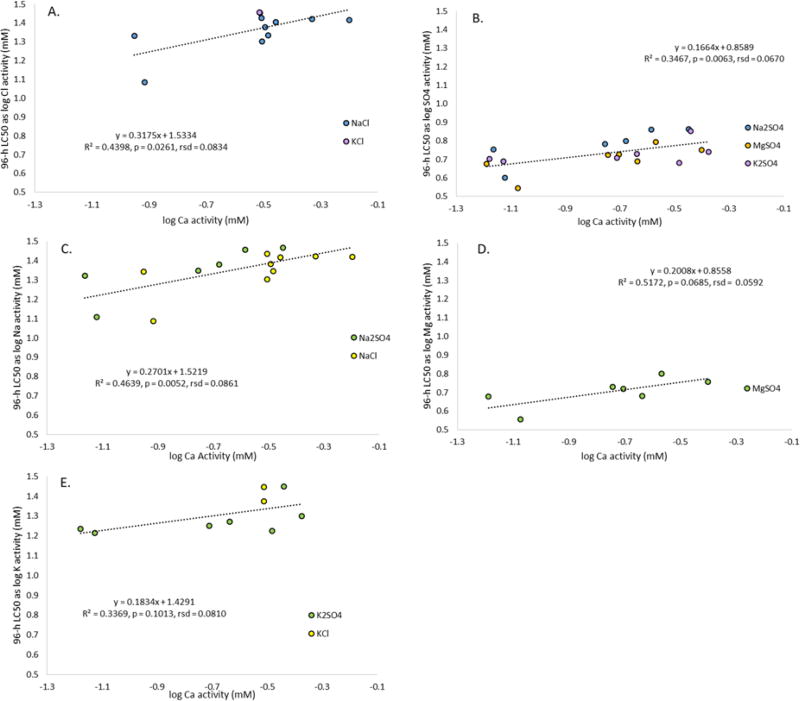
For all of the tests in the present study, we calculated LC50s and dilution water compositions in terms of activity (mM; Table S5) to account for the fact that, in more concentrated solutions, ions behave as though their concentrations are lower than their total concentrations [38]. The importance of considering activity in expressing exposure to major ion salts was further affirmed in analyses for C. dubia [9]. Determining specific causes of toxicity (e.g., anions, cations, osmolarity) in major ion salt tests was beyond the scope of the present study, but Erickson et al. [30] suggested the toxicities of K, Mg, and Ca to C. dubia appear attributable to the cations rather than anions, and that Na salt toxicity may be related to multiple ions or osmolarity (i.e., not necessarily the associated anion). Here, we report toxicity data for the mayfly in terms of both anion and cation activities plotted against Ca activity, because Ca was the most frequently manipulated major ion in dilution waters, and was shown to be a primary influence on toxicity of Na and Mg salts to C. dubia [9].
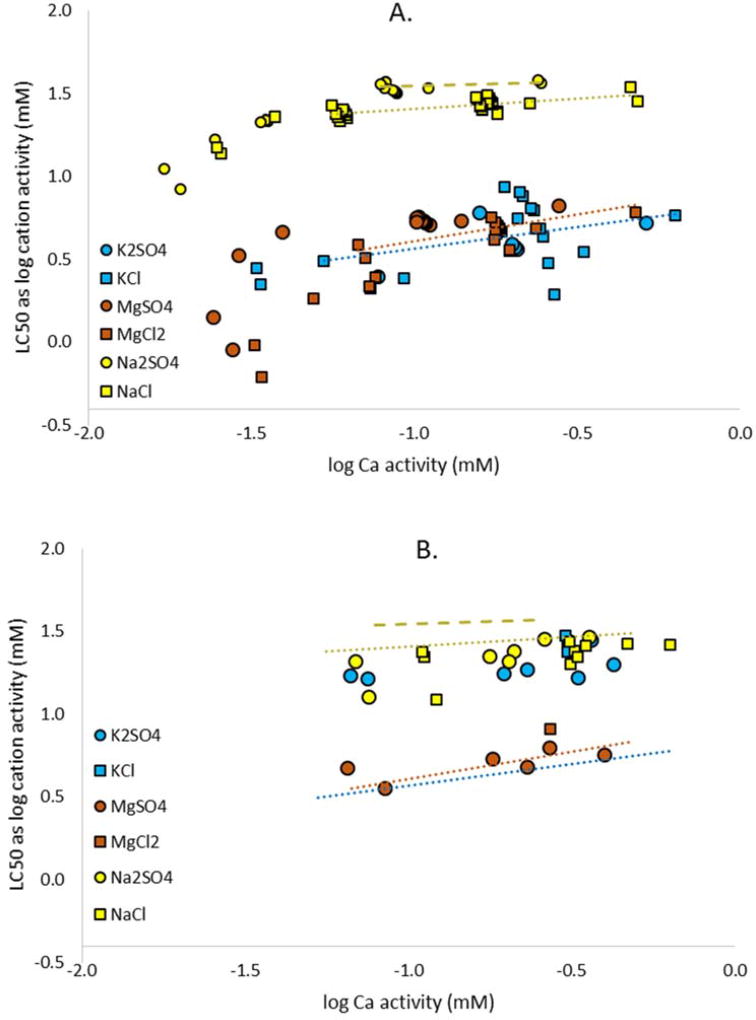
As shown in Figure 2, the 96-h LC50s for Cl, SO4, and Na were significantly positively correlated with Ca activity in the dilution water, while correlations for Mg and K with Ca were insignificant (p > 0.05). While not statistically different from zero because of the inclusion of fewer tests, the slope for the Mg data was similar to those of the other ions, and greater than the significantly positive slope for SO4, which included more than double the number of tests. The chloride plot had the steepest slope, followed closely by Na. Mount et al. [9] reported on the influence of Ca on Na salt toxicity (as Na activity) for C. dubia (Fig. 3), but that study tested waters with substantially lower Ca concentrations than the present study did (as low as 0.04 mM compared to our lowest value of 0.2 mM). Much of the steepness in the curve in the C. dubia study [9] was below 0.2 mM Ca. Over the comparable range of Ca activities, the responses of the two organisms are similar, and if we had tested N. triangulifer in waters with even lower Ca concentrations, we might have observed a similarly stronger Ca dependence of Na toxicity. It is notable that in the case of the C. dubia data [9] (Fig. 3), there is distinct separation on the y-axis between NaCl and Na2SO4; this was not observed for the mayfly.
Figure 2.
Influence of calcium activity on 96-h median lethal concentrations (LC50s) of various major ion salts for the mayfly Neocloeon triangulifer expressed as logs of A.) chloride activity, B.) sulfate activity, C.) sodium activity, D.) magnesium activity, and E.) potassium activity. RSD = residual standard deviation
Figure 3.
Influence of calcium activity on acute median lethal concentrations (LC50s) of various major ion salts for the A.) Ceriodaphnia dubia (from Mount et al. 2016) and B.) the mayfly Neocloeon triangulifer. All LC50s expressed in terms of cation activity for the given salt. Trendlines in panel A are linear fits for C. dubia data over the range of Ca activities tested with Neocloeon. The longer dashed yellow line fits Na2SO4 data, whereas the finer dotted line fits NaCl data. The same C. dubia trendlines are then superimposed with the Neocloeon data in panel B.
Although the Ca slope for Mg activity was not statistically separable from zero, the Mg data for mayfly are generally consistent with the Ca slope found for C. dubia [9], and, as for the Na salts, the strongest effect of Ca on Mg salt toxicity for C. dubia occurred at Ca activities lower than those tested here for mayfly (Fig.3). Another interesting trend apparent in Figure 3 is that the toxicity of Na and K are quite similar for N. triangulifer with Mg being substantially more toxic. This contrasts sharply with data for C. dubia and fathead minnows, for which K and Mg salts were substantially more toxic than Na salts [1, 9]. Unpublished data from C. Ivey et al. (2013, poster presented at Society of Environmental Toxicology and Chemistry) indicate an even greater disparity between Na and K toxicity to freshwater mussels, with KCl being on the order of 50 fold more toxic than NaCl on a mM basis.
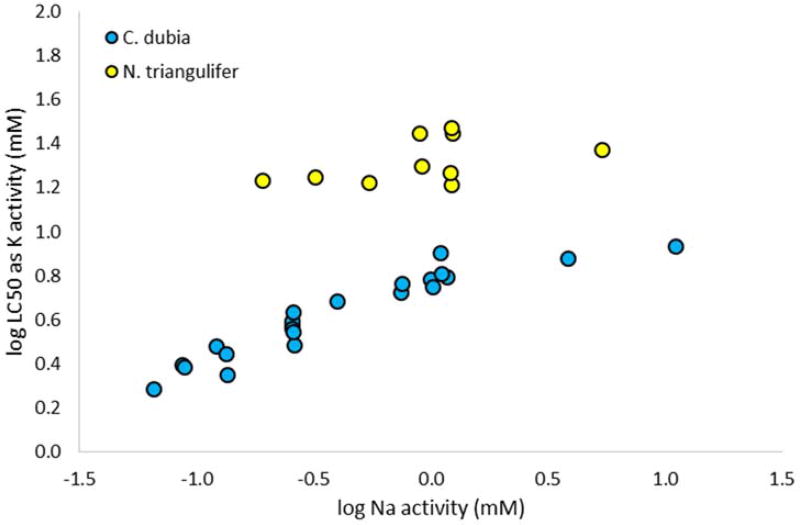
Figure 4 additionally plots K salt LC50s against Na activity in dilution water for comparison to the finding of Mount et al. [9] that Na regulates K salt toxicity to C. dubia. There is little slope evident in the data for the mayfly, further confirming our conclusion that in the case of this species, Na appears to have little influence on K salt toxicity.
Figure 4.
Potassium (K) salt median lethal concentrations (LC50s) for the mayfly Neocloeon triangulifer and the cladoceran Ceriodaphnia dubia (from Mount et al. 2016) as a function of sodium (Na) activity in dilution water.
Sensitivity of N. triangulifer relative to other organisms
For the sodium salts, the range of LC50s we observed for N. triangulifer indicate it is acutely sensitive relative to other species. The acute toxicity of NaCl in waters of 80 to 100 mg/L hardness has been reported for many aquatic species. The chloride LC50 for N. triangulifer (837 mg Cl/L at nominal hardness of 90, Table 1) in the present study is lower than those for eleven other species tested in this hardness range including cladocerans (C. dubia, Daphnia ambigua, D. magna) [1, 6, 12, 39], a mussel (L. siliquoidea) [37], a rotifer (Brachionus calyciflorus) [6], and an amphipod (H. azteca) [6, 40], two worms (Lumbriculus variegatus and Tubifex tubifex) [6], an insect (Chironomus dilutus) [6], and two fish (Pimephales promelas and Oncorhynchus mykiss) [1, 6]. Notably, the mayfly was more sensitive than C. dubia, for which there are a number of published LC50s in this hardness range (average LC50 ~ 1,138 mg Cl/L; [1, 6, 9, 12, 39]. Struewing et al. [24] and SWRC (J. Jackson, personal communication) have reported NaCl LC50s for N. triangulifer previously, but those tests were 48-h in duration compared to the 96-h tests in the present study, so comparisons of effect levels with our data are tenuous. In the present study, 48-h LC50s were consistently approximately double the corresponding 96-h LC50s (data not shown). The first molt for the organism occurs within this timeframe and might account for the sharp difference in 48-h and 96-h LC50s (personal observation DJS). The glochidia of three mussel species (Lampsilis fasciola, Epioblasma torulosa, and Lampsilis cardium) have been found to be more sensitive than N. triangulifer (LC50s ranging from 179 to 817 mg Cl/L; [37].
For Na2SO4, the LC50 for N. triangulifer at hardness = 90 mg/L in the present study (1,338 mg SO4/L, Table 1) was lower than those for the seven other species for which we found data at that approximate hardness, including Hyalella azteca, Sphaerium simile, Daphnia magna, Pimephales promelas, Chironomus dilutus, Lampsilis abrupta, and C. dubia [1, 5, 9–11, 13]. Reported LC50s for these species ranged from 1874 to 14,134 mg/L. Goetsch and Palmer [41] generated an LC50 of 500 mg SO4/L for the mayfly Tricorythus sp. at a lower hardness (~69 mg/L), confirming the sensitivity of mayflies to Na2SO4. There is a published data point at a similar hardness for H. azteca of 512 mg SO4/L [10], but the dilution water for that toxicity test had a chloride concentration lower than that considered sufficient for optimal health for that species [42]. Thus in general, N. triangulifer appears to be relatively sensitive to sodium salts, especially sodium sulfate.
Compared to the sodium salts, far fewer published data are available on the acute toxicity of Mg and K salts to other species [1, 9, 43]. The Mg salt toxicity data for C. dubia, D. magna, and P. promelas reported by Mount et al. [1] with LC50s ranging from 224 to 569 mg Mg/L, are similar to those reported here for the mayfly at a similar hardness (224 to 411 mg Mg/L at hardness ~90 mg/L, Table 1). Conversely, N. triangulifer was substantially less sensitive to K than the three species tested by Mount et al. [1], the former having LC50s ranging from 785 to 1,326 mg K/L at hardness ~90 mg/L (Table 1), and the latter having LC50s of <305 to 462 mg K/L. Van Dam et al. [43] tested the responses of a variety of Australian freshwater species from an area with background water containing extremely low Ca to MgSO4, with most having median effect concentrations between 4.4 and 63 mg Mg/L (the exception being Chlorella at 1,215 mg Mg/L). The lower values from that study are approximately an order of magnitude lower than those reported here (Table 1), but the dilution water in the Van Dam et al. [43] study had very low Ca (<0.8 mg/L), which could account for the difference given the effect of Ca on Mg toxicity observed in the present study.
CONCLUSIONS
In summary, the mayfly was in general relatively sensitive to NaCl, MgSO4, and especially Na2SO4 compared to other species reported in the literature, but relatively less so to K salts. Sodium and magnesium salt toxicity decreased with increased hardness while the K salt toxicity did not, consistent with findings for other species. However, in Ca:Mg manipulations, it is not clear whether Ca activity alone can account for all of the “hardness” effect. Over the range of Ca activity tested for the mayfly, the slopes of the Ca effect on toxicity of Na and Mg salts to mayfly were similar to those reported elsewhere for C. dubia, though it is unclear whether the steeper slopes reported for C. dubia and lower Ca activities would also occur for N. triangulifer. Two other key differences between these two species were 1) that K salt toxicity to N. triangulifer was not modified by Na in dilution water, and 2) that K toxicity to mayflies was very similar to Na toxicity on a mM basis, instead of the much greater toxicity of K observed for C. dubia.
Supplementary Material
Acknowledgments
We thank R. Erickson, US Environmental Protection Agency, Mid-Continent Ecology Division, for consulting on experimental design and analysis. D. Glazik (University of Illinois) assisted with toxicity testing. J. Jackson, Stroud Water Research Center provided the unpublished SWRC data referred to throughout the text. This study was funded by the Great Lakes Restoration Initiative by way of a Cooperative Ecosystem Studies Unit grant from US Geological Survey, Columbia Environmental Research Center. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- 1.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows) Environ Toxicol Chem. 1997;16:2009–2019. [Google Scholar]
- 2.Goodfellow WL, Ausley LW, Burton DT, Denton DL, Dorn PB, Grothe DR, Heber MA, Norberg-King TJ, Rodgers JH., Jr Major ion toxicity in effluents: A review with permitting recommendations. Environ Toxicol Chem. 2000;19:175–182. [Google Scholar]
- 3.Corsi SR, Graczyk DJ, Geis SW, Booth NL, Richards KD. A fresh look at road salt: aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ Sci Technol. 2010;44:7376–7382. doi: 10.1021/es101333u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer MA, Bernhardt ES, Schlesinger WH, Eshleman KN, Foufoula-Georgiou E, Hendryx MS, Lemly AD, Likens GE, Loucks OL, Power ME, White PS, Wilcock PR. Mountaintop mining consequences. Science. 2010;327:148–149. doi: 10.1126/science.1180543. [DOI] [PubMed] [Google Scholar]
- 5.Davies TD, Hall KJ. Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ Toxicol Chem. 2007;26:1243–1247. doi: 10.1897/06-510r.1. [DOI] [PubMed] [Google Scholar]
- 6.Elphick JR, Bergh KD, Bailey HC. Chronic toxicity of chloride to freshwater species: Effects of hardness and implications for water quality guidelines. Environ Toxicol Chem. 2011a;30:239–246. doi: 10.1002/etc.365. [DOI] [PubMed] [Google Scholar]
- 7.Elphick JR, Davies M, Gilron G, Canaria EC, Lo B, Bailey HC. An aquatic toxicological evaluation of sulfate: The case for considering hardness as a modifying factor in setting water quality guidelines. Environ Toxicol Chem. 2011b;30:247–253. doi: 10.1002/etc.363. [DOI] [PubMed] [Google Scholar]
- 8.Lasier PJ, Hardin IR. Observed and predicted reproduction of Ceriodaphnia dubia exposed to chloride, sulfate and bicarbonate. Environ Toxicol Chem. 2010;29:347–358. doi: 10.1002/etc.29. [DOI] [PubMed] [Google Scholar]
- 9.Mount DR, Erickson RJ, Highland TL, Hockett JR, Hoff DJ, Jenson CT, Norberg-King TJ, Peterson KN, Polaske ZM, Wisniewski S. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environ Toxicol Chem. 2016;35:3039–3057. doi: 10.1002/etc.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soucek DJ, Kennedy AJ. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ Toxicol Chem. 2005;24:1204–1210. doi: 10.1897/04-142.1. [DOI] [PubMed] [Google Scholar]
- 11.Soucek DJ. Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ Toxicol Chem. 2007;26(4):773–779. doi: 10.1897/06-229r.1. [DOI] [PubMed] [Google Scholar]
- 12.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ Toxicol Chem. 2011;30:930–938. doi: 10.1002/etc.454. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Dorman RA, Ingersoll CG, Hardesty DK, Brumbaugh WG, Hammer EJ, Bauer CR, Mount DR. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ Toxicol Chem. 2016;35:115–127. doi: 10.1002/etc.3148. [DOI] [PubMed] [Google Scholar]
- 14.Boehme EA, Zipper CE, Schoenholtz SH, Soucek DJ, Timpano AJ. Temporal dynamics of benthic macroinvertebrate communities and their response to elevated specific conductance in Appalachian coalfield headwater streams. Ecol Indic. 2016;64:171–180. [Google Scholar]
- 15.Cormier SM, Suter GW, Zheng L. Derivation of a benchmark for freshwater ionic strength. Environ Toxicol Chem. 2013;32:263–271. doi: 10.1002/etc.2064. [DOI] [PubMed] [Google Scholar]
- 16.Pond GJ, Passmore ME, Borsuk FA, Reynolds L, Rose CJ. Downstream effects of mountaintop coal mining: comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. J N Am Benthol Soc. 2008;27:717–737. [Google Scholar]
- 17.Pond GJ. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA) Hydrobiologia. 2010;641:185–201. [Google Scholar]
- 18.Timpano AJ, Schoenholtz SH, Soucek DJ, Zipper CE. Salinity as a limiting factor for biological condition in mining-influenced Central Appalachian headwater streams. J Am Water Resour As. 2015;51:240–250. [Google Scholar]
- 19.Hassell KL, Kefford BJ, Nugegoda D. Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae) J Exp Biol. 2006;209:4024–4032. doi: 10.1242/jeb.02457. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BR, Weaver PC, Nietch CT, Lazorchak JM, Struewing KA, Funk DH. Elevated major ion concentrations inhibit larval mayfly growth and development. Environ Toxicol Chem. 2015;34:167–172. doi: 10.1002/etc.2777. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AJ, Cherry DS, Currie RJ. Evaluation of ecologically relevant bioassays for a lotic system impacted by a coal-mine effluent, using Isonychia. Environ Monit Assess. 2004;95:37–55. doi: 10.1023/b:emas.0000029896.97074.1e. [DOI] [PubMed] [Google Scholar]
- 22.Kunz JL, Conley JM, Buchwalter DB, Norberg-King TJ, Kemble NE, Wang N, Ingersoll CG. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ Toxicol Chem. 2013;32:2826–2835. doi: 10.1002/etc.2391. [DOI] [PubMed] [Google Scholar]
- 23.Soucek DJ, Dickinson A. Full-life chronic toxicity of sodium salts to the mayfly Neocloeon triangulifer in tests with laboratory cultured food. Environ Toxicol Chem. 2015;34(9):2126–2137. doi: 10.1002/etc.3038. [DOI] [PubMed] [Google Scholar]
- 24.Struewing KA, Lazorchak JM, Weaver PC, Johnson BR, Funk DH, Buchwalter DB. Part 2: Sensitivity comparisons of the mayfly Centroptilum triangulifer to Ceriodaphnia dubia and Daphnia magna using standard reference toxicants; NaCl, KCL and CuSO4. Chemosphere. 2105;139:597–603. doi: 10.1016/j.chemosphere.2014.04.096. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney BW, Funk DH, Standley LJ. Use of the stream mayfly Cloeon triangulifer as a bioassay organism: life history response and body burden following exposure to technical chlordane. Environ Toxicol Chem. 1993;12:115–125. [Google Scholar]
- 26.Weaver PC, Lazorchak JM, Struewing KA, DeCelles SJ, Funk DH, Buchwalter DB, Johnson BR. Part 1: Laboratory culture of Centroptilum triangulifer (Ephemeroptera:Baetiae) using a defined diet of three diatoms. Chemosphere. 2015;139:589–596. doi: 10.1016/j.chemosphere.2014.04.092. [DOI] [PubMed] [Google Scholar]
- 27.Zalizniak L, Kefford B, Nugegoda D. Is all salinity the same? I. the Effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Mar Freshwater Res. 2006;57:75–82. [Google Scholar]
- 28.Clements WH, Kotalik C. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw Sci. 2016;35:126–138. [Google Scholar]
- 29.Kefford BJ, Papas PJ, Nugegoda D. Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Mar Freshwater Res. 2003;54:755–765. [Google Scholar]
- 30.Erickson RJ, Mount DR, Highland TL, Hockett JR, Hoff DJ, Jenson CT, Norberg-King TJ, Peterson KN. The acute toxicity of major ion salts to Ceriodaphnia dubia. II. Empirical relationships in binary salt mixtures. Environ Toxicol Chem. 2016;36:1525–1537. doi: 10.1002/etc.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDunnough J. New species of North American Ephemeroptera. Can Entomol. 1931;63:82–93. [Google Scholar]
- 32.McCafferty W, Waltz R. Revisionary synopsis of the Baetidae (Ephemeroptera) of North and Middle America. Trans Am Entomol Soc (Phi) 1990;116:769–799. [Google Scholar]
- 33.Jacobus LM, Wiersema NA. The genera Anafroptilum Kluge, 2011 and Neocloeon traver, 1932, reinstated status, in North America, with remarks about global composition of Centroptilum Eaton, 1869 (Ephemeroptera:Baetidae) Zootaxa. 2014;3814(3):385–391. doi: 10.11646/zootaxa.3814.3.5. [DOI] [PubMed] [Google Scholar]
- 34.ASTM E729-96. Standard guide for conducting acute toxicity testing on test materials with fishes, macroinvertebrates, and amphibians. ASTM International; West Conshohocken, PA: 2014. [Google Scholar]
- 35.APHA (American Public Health Association), American Water Works Association and Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 21. American Public Health Association; Washington DC: 2005. [Google Scholar]
- 36.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating lethal concentrations in toxicity bioassays. Environ Sci Technol. 1977;1:714–719. [Google Scholar]
- 37.Gillis PL. Assessing the toxicity of sodium chloride to the glochidia of freshwater mussels: Implications for salinization of surface waters. Environ Pollut. 2011;159:1702–1708. doi: 10.1016/j.envpol.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Snoeyink VL, Jenkins D. Water Chemistry. John Wiley & Sons; New York, NY: 1980. [Google Scholar]
- 39.Harmon SM, Specht WL, Chandler GT. A comparison of the daphnids Ceriodaphnia dubia and Daphnia ambigua for the utilization in routine toxicity testing in the southeastern United States. Arch Environ Contam Toxicol. 2003;45:79–85. doi: 10.1007/s00244-002-0116-8. [DOI] [PubMed] [Google Scholar]
- 40.Soucek DJ, Dickinson A, Major KM, McEwen AR. Effect of test duration and feeding on relative sensitivity of genetically distinct clades of Hyalella azteca. Ecotoxicology. 2013;22:1359–1366. doi: 10.1007/s10646-013-1122-5. [DOI] [PubMed] [Google Scholar]
- 41.Goetsch PA, Palmer CG. Salinity tolerances of selected macroinvertebrates of the Sabie River, Kruger National Park, South Africa. Arch Environ Contam Toxicol. 1997;32:32–41. doi: 10.1007/s002449900152. [DOI] [PubMed] [Google Scholar]
- 42.Soucek DJ, Mount DR, Dickinson A, Hockett JR, McEwen AR. Contrasting effects of chloride on growth, reproduction, and toxicant sensitivity in two genetically distinct strains of Hyalella azteca. Environ Toxicol Chem. 2015;34:2354–2362. doi: 10.1002/etc.3070. [DOI] [PubMed] [Google Scholar]
- 43.Van Dam RA, Hogan AC, McCullough CD, Houston MA, Humphrey CL, Harford AJ. Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environ Toxicol Chem. 2010;29:410–421. doi: 10.1002/etc.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.