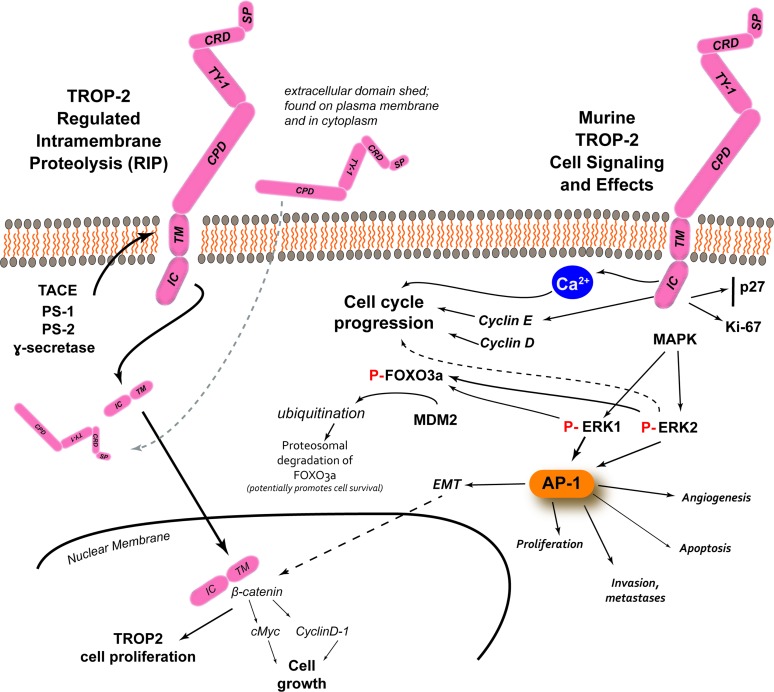
Figure 2. TROP-2 processing, cell signaling and its effects [adapted from Shvartsur and Bonavida, [9]].
In prostate cancer, studies have found several enzymes involved in the cleavage of the TM-IC portion of the molecule, with the extracellular domain remaining associated with the plasma membrane or found in the cytoplasm. β-catenin colocalized with TM-IC in the nucleus, which can lead to TROP-2-driven proliferation, but also it can upregulate cyclin D1 and c-myc, which can lead to cell growth. Apart from its processing, TROP-2 has the potential to influence several intracellular signaling pathways that can then lead to several different events. The phosphorylation of serine-303 appears be involved in the release intracellular Ca2+, which can activate Raf and NF-κB pathways, and stimulate MAPK signaling and cell cycle progression. TROP-2 can increase cyclin D1 and cyclin E, which together with ERK1/2 can mediate cell cycle progression. Studies with murine TROP-2 have revealed the stimulation of MAPK and downstream upregulation of phosphorylated ERK1/2 can induce the AP-1 transcription factor that can regulate a number of tumor-associated target genes involved in angiogenesis (e.g., via VEGF), proliferation (e.g., via cyclins and CDKs), apoptosis (e.g., via BCL-2, FasL), and invasion and metastasis (e.g., via matrix metalloproteinases, podoplanin, Ezrin, and CD33), as well as epithelial to mesenchymal transition (EMT) that can interact with β-catenin to affect cell growth. Studies with murine TROP-2 also found that increased ERK activity can induce the phosphorylation of FOXO3a, followed by its ubiquitination by mouse double minute 2 (MDM2), with its subsequent degradation. The degradation of FOXO3a can promote cancer cell survival.