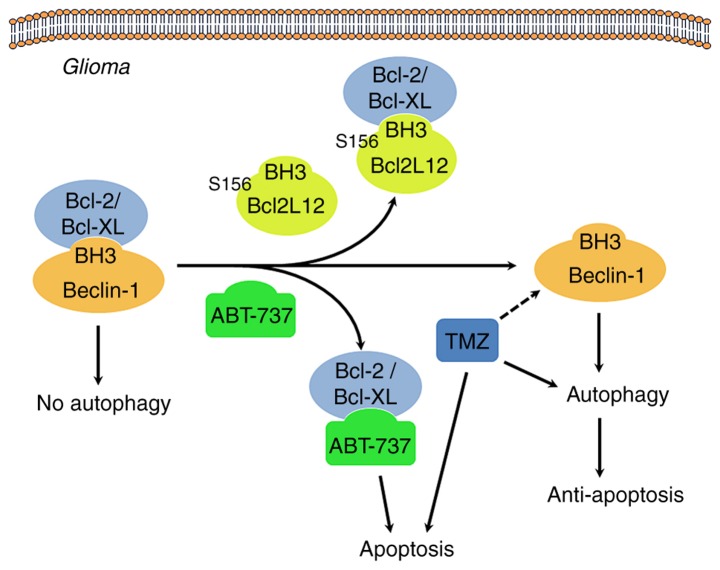
Figure 9.
Proposed mechanism of Ser156 phosphorylation as an allosteric site to modulate a BH3-like domain on BCL2L12 in glioma cells. When BCL2/BCL-XL interacts with the Beclin-1 BH3 domain, autophagy is inhibited. Overexpression of BCL2L12 may displace Beclin-1 in integrating with BCL2/BCL-XL via its BH3-like domain, leading to release of Beclin-1 and initiation of the autophagy process. In addition, since BCL2L12 occupies the hydrophobic groove of BCL2/BCL-XL, BH3 only BCL2 activator or sensitizer is unable to gain access, and the gross result of anti-apoptosis is observed. The BH3 domain mimetic agent, ABT-737, also binds to BCL2/BCL-XL, and hence competes and disrupts the interaction between BCL2/BCL-XL and BCL2L12, making tumor cells more vulnerable to apoptosis. Of note, GSK3β-mediated BCL2L12 S156 phosphorylation may affect BH3 domain function in glioma cells. BCL2L12, BCL2-like 12; BCL2, BCL2 apoptosis regulator; BCL-XL, BCL-extra large; GSK3β, glycogen synthase kinase 3β.