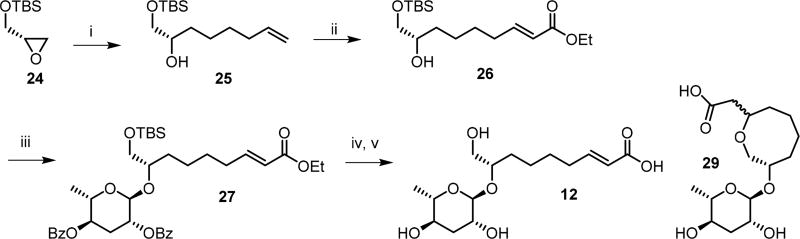
Scheme 4. Synthesis of the (ω)-Hydroxyacyl Ascaroside asc-9OH-ΔC9 (12).
Reagents and conditions: (i) 4-pentenylmagnesium bromide, copper(I)iodide, THF, 0 °C, 3 h, 94%; (ii) ethyl acrylate, Grubb’s second generation catalyst, CH2Cl2, 40 °C, 9 h, 81%; (iii) 2,4-di-O-benzoylascarosyl-1-(2,2,2-trichloroacetimidate) (21), trimethylsilyl triflate, CH2Cl2, 0 °C, 3 h, 53%; (iv) tetrabutylammonium fluoride, THF, 3 h, 78%; (v) lithium hydroxide, water, MeOH, 12 h, 25% (12) and 69% (29).