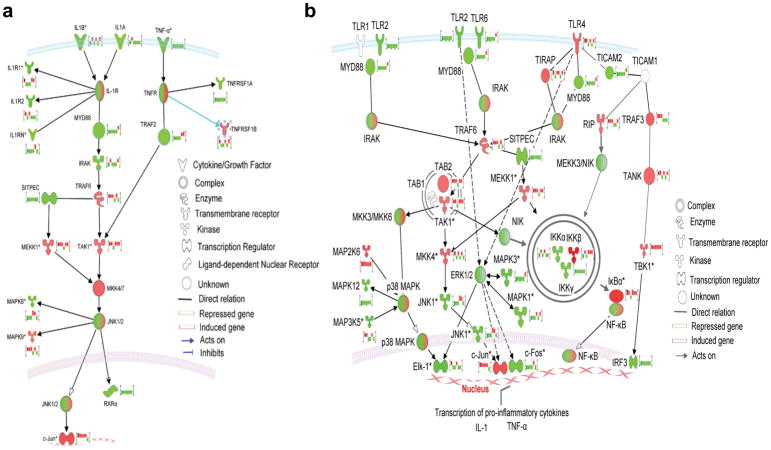
Figure 3.
(a) Modulation of interleukein-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) pathways by a selective CB2 agonist, 1-(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl) piperidine (MDA7) (generated by Ingenuity pathway analysis using comparison analysis between the 6 arrays). The brightness of node colors is proportional to the fold changes of gene expression levels. Color indicates up-regulated (red) and down-regulated (green) genes. In addition fold change expression bar charts of the six arrays have been included for each gene. In the IL-1 pathway, a complex is formed including IL-1R-associated kinases (IRAK) and the adapter protein MyD88. IRAK is rapidly phosphorylated and associates with TNF receptor-associated factor 6 (TRAF6); this association is necessary for downstream IL-1-induced translocation of signaling molecules to the nucleus, which ultimately leads to expression of genes that mediate inflammation and, frequently, tissue destruction. MDA7 acts by inhibiting IL-1 and TNF-α decreased expression of MyD88 and TNF receptor-associated factor 2 (TRAF2). (b) Signaling cascades initiated via toll-like receptor 2 (TLR2)- and TLR4-dependent activation and its modulation by MDA7. In addition fold change expression bar charts of the six arrays have been included for each gene. Engagement of TLR2 on the cell surface as a heterodimer with either TLR1 or TLR6 leads to recruitment of the adaptor protein MyD88 and interaction with TIR-domain-containing adaptor protein (TIRAP) via death-domain interactions. Phosphorylated IL-1 receptor-associated kinase (IRAK), together with TNF receptor-associated factor 6 (TRAF6), dissociates from the receptor, and then TRAF6 interacts with TAK1 binding protein 1 (TAB1)—induces autophosphorylation of the transforming growth factor β (TGFβ)-activating kinase (TAK1), and TAB2. This leads to phosphorylation of the IκBα-kinase (IKK) complex (IKKα, IKKβ, and IKKγ) and mitogen-activated protein kinases (MAPK), such as c-Jun NH2-terminal kinase (JNK), inducing the nuclear translocation of NF-κB and subsequent induction of target genes such as TNFα and ILs. The transcriptional activity of NF-κB is tightly regulated by its association with the inhibitory IκB that sequesters NF-κB in the cytosol. Beneficial effects of MDA7 include modulation of general genes that will ultimately inhibit phosphorylation of IκB proteins by the IKKs and prevention of IκB degradation. TLR4-MyD88-independent pathway activation involves signaling through the Toll-interleukin-1 receptor (TIR) adaptor TRIF (also known as TICAM1), TRIF-related adaptor molecule (TRAM; also known as TICAM2), TRAF3, receptor-interacting protein (RIP) and the transcription factor interferon regulatory factor 3 (IRF3). The green and red bar charts and shading represent the respective differential gene modulation (repression or induction, respectively) by MDA7. The numbers represent fold change. (Reproduced from Xu et al.[73] with permission).