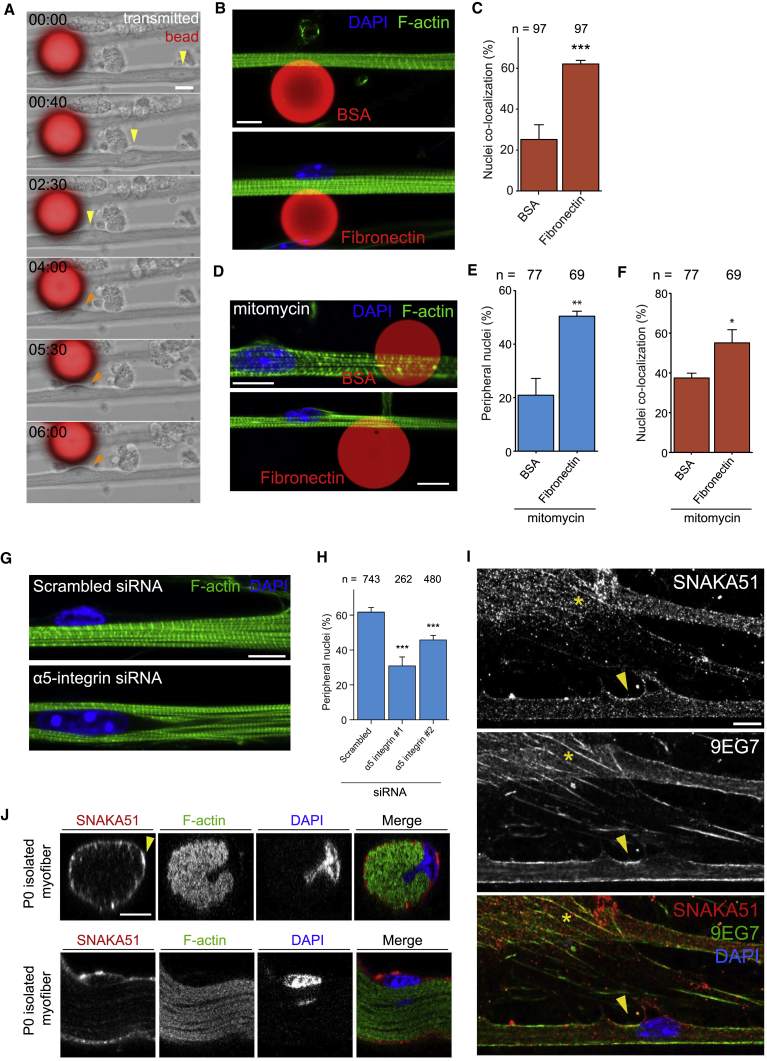
Figure 3.
Local Accumulation of Fibronectin Triggers Peripheral Nuclear Positioning via α5-Integrin
(A) Time-lapse (hr:min) images of myofiber cultures (transmitted) 20-μm beads coated with fibronectin (fluorescence, red). Note that centrally located nucleus (yellow arrowhead) moves toward the bead and to the periphery once it reaches the bead (orange arrowhead). Scale bar, 10 μm.
(B) Representative immunofluorescence z-projection images of 10-day myofibers cultured with 20-μm beads coated with either BSA (top) or fibronectin (bottom) and stained for F-actin (myofibrils, green) and DAPI (nucleus, blue). Scale bar, 10 μm.
(C) Quantification of number of nuclei from 10-day myofibers in proximity to BSA- or fibronectin-coated beads. Data from three independent experiments were combined and error bars represent SEM from indicated number of nuclei (n) for each cohort. Unpaired t test was used to determine statistical significance, where ∗∗∗p < 0.001.
(D) Representative immunofluorescence z-projection images of 10-day myofibers treated with mitomycin and cultured with 20-μm beads coated with either BSA or fibronectin and stained for F-actin (myofibrils, green) and DAPI (nucleus, blue). Scale bar, 10 μm.
(E) Quantification of number of peripheral nuclei from 10-day myofibers treated with mitomycin and in contact with BSA- or fibronectin-coated beads. Data from three independent experiments were combined and error bars represent SEM from indicated number of nuclei (n) for each cohort. Unpaired t test was used to determine statistical significance, where ∗∗p < 0.01.
(F) Quantification of number of nuclei from 10-day myofibers treated with mitomycin in proximity to BSA- or fibronectin-coated beads. Data from three independent experiments were combined and error bars represent SEM from indicated number of nuclei (n) for each cohort. Unpaired t test was used to determine statistical significance, where ∗p < 0.05.
(G) Representative immunofluorescence image of 10-day myofibers knocked down for scrambled or α5-integrin and stained for F-actin (myofibrils, green) and DAPI (nucleus, blue). Scale bar, 10 μm.
(H) Quantification of peripheral nuclei in 10-day myofibers knocked down for scrambled or α5-integrin. Data from three independent experiments were combined and error bars represent SEM from indicated number of nuclei (n) for each cohort. Unpaired t test was used to determine statistical significance, where ∗∗∗p < 0.001.
(I) Representative immunofluorescence image of 5-day myofibers stained for SNAKA51 (α5-integrin, red), 9EG7 (β-integrin, green), and DAPI (nucleus, blue). Yellow arrowhead indicates α5-integrin activation in myofibers whereas yellow asterisk indicates fibrillar adhesions in myofibroblasts. Scale bar, 10 μm.
(J) Representative orthogonal (top) and side (bottom) immunofluorescence image of isolated myofibers from newborn mice stained for F-actin (myofibrils, green), SNAKA51 (α5-integrin, red), and DAPI (nucleus, blue). Yellow arrowhead indicates α5-integrin accumulation. Scale bar, 10 μm.