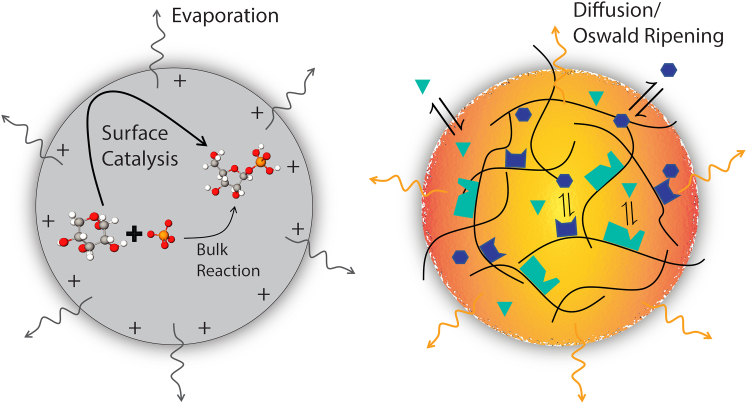
Figure 1.
Comparison of reactions within micro- and cellular condensates. The left schematic shows a microdroplet accelerating the production of ribose-1-phosphate from ribose and phosphate (48). The reaction is thought to be accelerated by the affinity of the polar reagents for the charged droplet interface, which localizes and orients the molecules. The right schematic depicts a cellular condensate composed of a scaffolding of multivalent RNPs. The scaffolding has a high density of enzymatic sites that interact with diffusing client molecules. The client molecules are able to readily cross the interface of the condensed phase and are more mobile in the droplet interior than the RNP scaffolding. Whereas microdroplets lose solvent through evaporation, thereby concentrating the reactants, cellular condensates exchange mass through Ostwald ripening, which could similarly have a concentrating effect. Lastly, whereas the air-water interface of the microdroplet is well defined, the interface of the cellular condensate is more diffuse and has thus far been less well-characterized. To see this figure in color, go online.