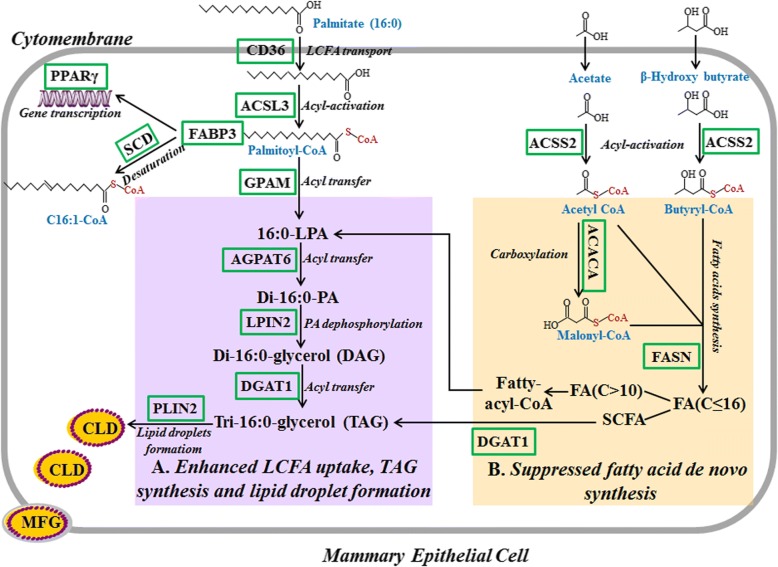
Fig. 5.
Scheme summarizing interrelationships among cellular pathways regulating lipid synthesis by palmitate in pMECs. a Palmitate enhanced the uptake of exogenous LCFA, TAG synthesis, and lipid droplet formation. Uptake of LCFA in pMECs, exampled by palmitate (16:0), was enhanced by palmitate through activating transport proteins (mainly CD36). Cytosolic 16:0 is converted into its activated form (16:0-CoA), with the help of ACSL. Cytosolic 16:0-CoA is transported to endoplasmic reticulum membrane by FABP and esterified there to glycerol-3-phosphate to produce 16:0-LPA by GPAM. In endoplasmic reticulum, addition of a second 16:0-CoA produces di-16:0-PA; di-16:0-PA can be hydrolyzed with LPIN to form a di-16:0-glycerol (DAG). The sn-3 position of DAG is then acylated to form TAG by DGAT. Newly formed TAG forms cytoplasmic lipid droplet in the ER membrane via incorporation. Then, the cytoplasmic lipid droplet was transported to the apical membrane and eventually released. b Palmitate suppressed the fatty acid de novo synthesis. In mammary cell, short- and medium-chain fatty acids (almost all C4:0~C14:0 and approximately 50% of palmitic acid) were highly dependent on the de novo synthesis. A series of cytosolic enzymes are required to facilitate this process, of which FASN and ACACA are considered the crucial enzymes of cellular fatty acid de novo synthesis in the porcine mammary gland. ACACA carboxylates acetyl-CoA to form malonyl-CoA, which is further converted by FASN to fatty acids (C ≤ 16). Then, the synthesized fatty acids participate in the TAG formation in endoplasmic reticulum