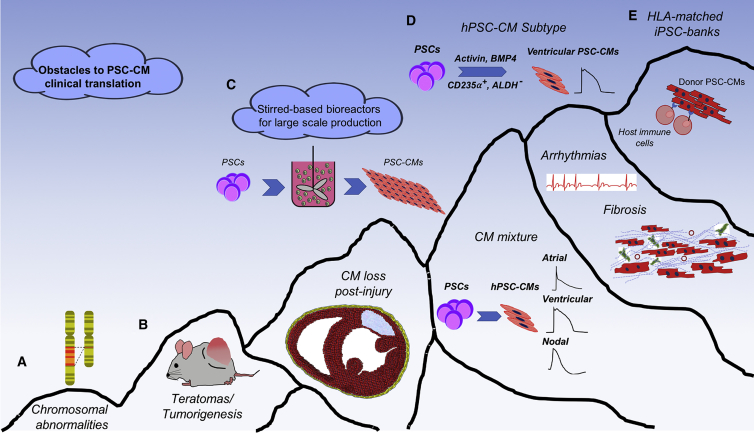
Figure 2.
Illustration of the “Mountain” of Obstacles to Overcome in Clinical Translation of PSC-CMs in Heart Disease
(A) Careful screening of PSC lines and state-of-the-art methodologies for the expansion and maintenance of PSCs are required to avoid genomic instability. (B) Engineering novel ways to eliminate undifferentiated PSCs from PSC derivatives can prevent teratoma formation. (C) To produce clinically relevant numbers of PSC-CMs, novel ways of PSC-CM manufacturing need to be developed. Advances in stirred-based bioreactors have allowed the maintenance and expansion of PSCs as well as the generation of PSC-CMs in a large scale that is sufficient to meet clinical demands. (D) The latest improvements in cardiac differentiation protocols toward specific cardiac subtypes will tremendously minimize the risk of arrhythmias caused by injection of mixed pools of PSC-CMs in fibrotic areas of the myocardium. (E) Creation of HLA-matched iPSC banks and engineering of immunoprivileged PSC-CMs will greatly reduce the risk of immune rejection of the grafted cells. HLA, human leukocyte antigen.