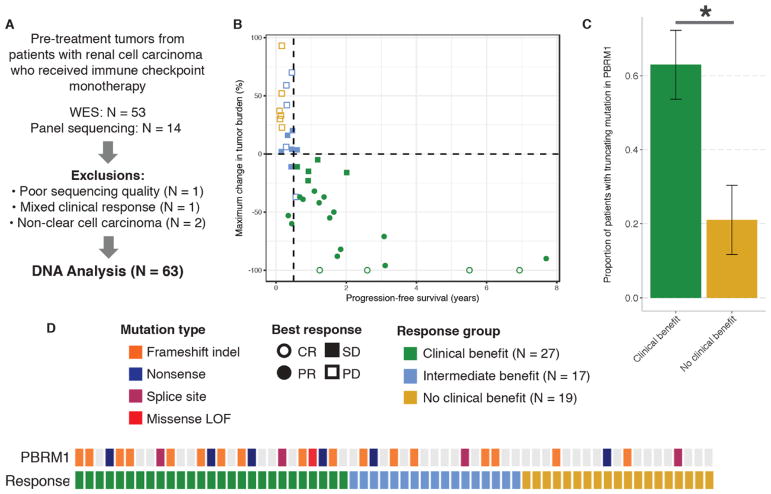
Fig. 3. PBRM1 LOF mutations correlate with clinical benefit in a validation cohort of ccRCC patients treated with immune checkpoint inhibitors.
(A) Selection of the validation cohort. (B) Clinical outcomes in the validation cohort. Ten patients without post-treatment re-staging scans (eight with clinical PD, two with SD, and one with PR) as well as 14 patients with targeted panel sequencing are not shown. (C) Proportion of tumors harboring PBRM1 LOF mutations in patients in the CB vs. NCB groups. Error bars are S.E. *Fisher’s exact p<0.05. (D) Truncating alterations in PBRM1 and response to anti-PD-(L)1 therapies by sample. Colored boxes indicate samples with truncating mutations in PBRM1 while gray denotes samples without PBRM1 truncating mutations. Missense LOF denotes a missense mutation detected by targeted sequencing that was confirmed to be LOF by PBRM1 immunohistochemistry (see Supplemental Methods).