Fig. 5.
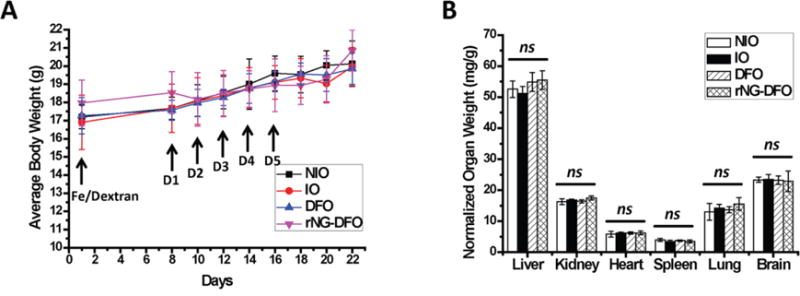
Female Balb/C mice were IO by single tail vein injection of Fe/Dextran (150 mg/kg of Fe) on Day 1; treatment with 150 mg/kg of DFO or equivalent rNG-DFO began on Day 8 and was repeated every other day for a total of 5 doses; NIO and untreated IO mice were injected with saline; mice were necropsied 7 days after the last dose. (A) BW of mice receiving rNG-DFO and DFO treatments remained within the normal range of ±15% throughout the duration of the study. (B) There were no acute signs of toxicity based on normalized organ weights with respect to animal body weight (mg/g). Results are presented as mean ± SD (n = 3). “ns” means the difference was not significant.
