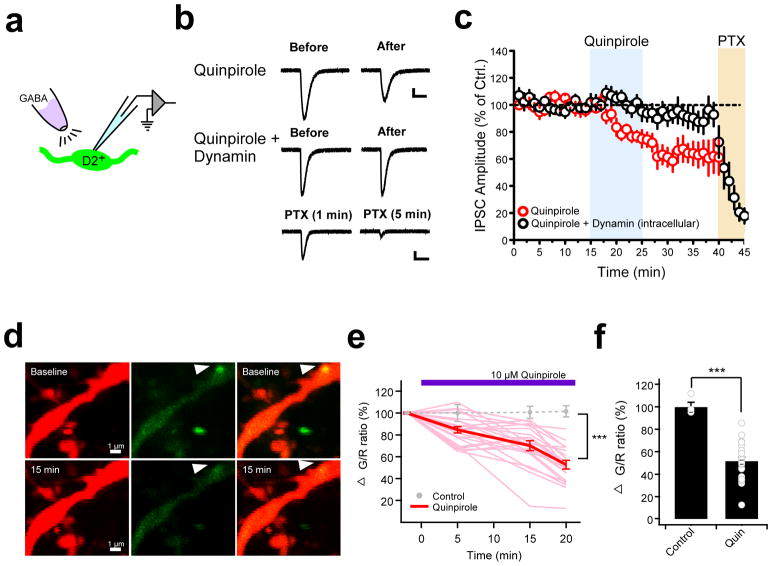
Figure 3. Postsynaptic mechanisms contribute to stress-induced inhibitory plasticity in the pPVT.
a. Schematic showing GABA puff experiment assessing postsynaptic involvement in quinpirole-induced GABAergic plasticity in D2+ neurons of the pPVT. b. Representative GABA-evoked responses from before and after bath application of quinpirole in the absence (top) and presence (middle) of intracellular dynamin. c. Average plot depicting the effect of quinpirole on GABA-evoked responses (n = 9, 6 mice). Note that the effect of quinpirole is prevented in neurons in which the dynamin inhibitory peptide (1 μM) was dialyzed through the patch pipette (n = 9, 4 mice). Bath application of picrotoxin (PTX) confirmed that postsynaptic responses were mediated by GABAA receptors. d. Representative two-photon images of a pPVT neuron dendrite expressing tdTomato (red, left) and Teal-gephyrin (green, middle) in both the absence (baseline, upper) and the presence (15 min, bottom) of 10 μM quinpirole. Arrowhead indicates a gephyrin-positive punctum whose fluorescence decreases following bath application of quinpirole. e. Changes in green/red (G/R) ratio at gephyrin puncta over time (light red, individual traces with quin; red, averaged trace with quin; gray circles, average without quinpirole). Difference between control and quinpirole: at 5 min P=0.051, at 15 min P=0.0046, and at 20 min P=0.000012; two-sided t-test. f. Summary bar graph of the change of G/R ratio before and 20 min after 10 μM quinpirole (Control: 100 ± 0.8%, n = 4 cells, 4 mice; Quinpirole: 52.8 ± 4.1%, n = 23 cells, 10 mice; ***P= 0.000012; two-sided t-test). Data shown as mean ± s.e.m.