Figure 7. FIGURE 7: Ways in which Rif1 dimerization may promote DSB repair and shape telomere architecture.
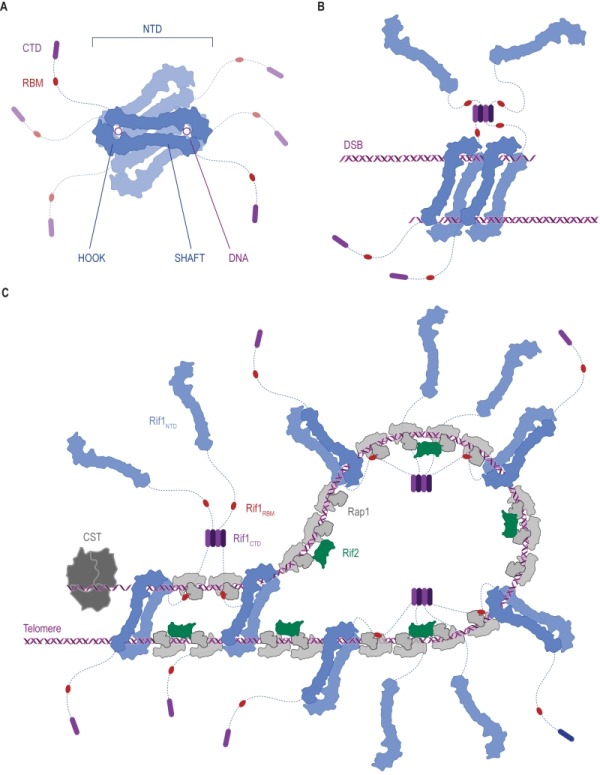
(A) Crystallographic model showing budding yeast Rif1 dimers bound to two DNA molecules 19. The shepherd’s crook-like Rif1NTD (blue) comprises an N-terminal HOOK and a straight SHAFT region. Rif1RBM (Rap1-binding domain, red) and Rif1CTD (C-terminal tetramerization domain, purple) are connected to Rif1NTD by unstructured linker regions (dotted lines). Rif1NTD has intrinsic DNA-binding activity and assembles on DNA as a figure 8-shaped, head-to-tail dimer. Multiple dimers may be organized around the same DNA molecules, forming a protein sheath, and restricting access of other proteins.
(B) Speculative model of Rif1 dimers binding to the two ends of a DSB: tethering DSB ends in this way may promote re-ligation along the NHEJ pathway by keeping DSB ends in close proximity.
(C) Schematic representation of the Velcro-like protein network formed by Rap1, Rif1 and Rif2 at yeast telomeres, taking DNA binding by Rif1 into account. Rap1 molecules (grey) directly bind dsDNA TG1-3 tracts, recruiting Rif1 and Rif2. Rif2 (green) interlinks adjacent Rap1 molecules, while Rif1, forming tetramers via its CTD, engages multiple Rap1 molecules through RBM epitopes. The NTD may allow Rif1 to directly engage telomeric DNA at sites not covered by Rap1. Fold-back structures could potentially be stabilized by Rif1-mediated DNA-bridging.
