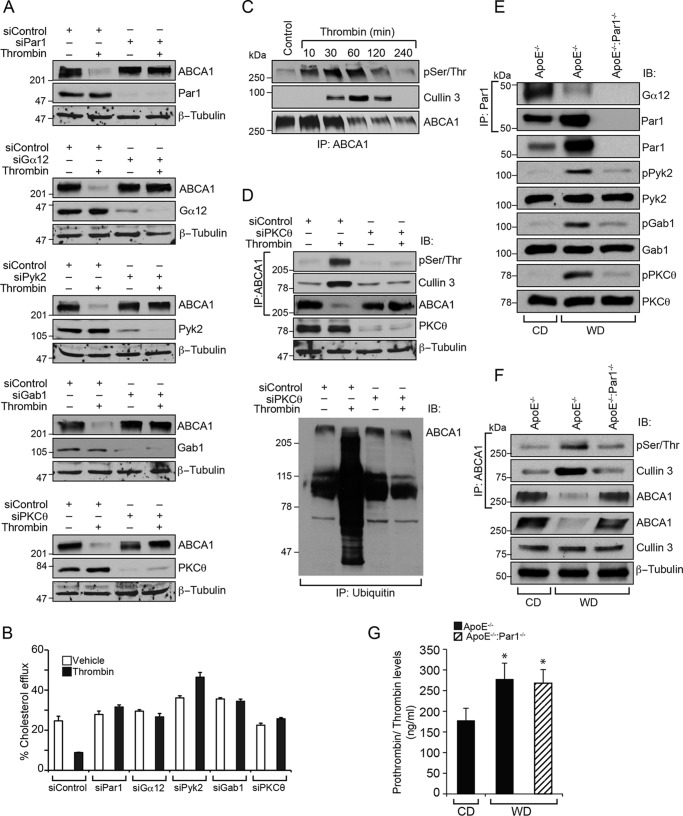
Figure 8.
PKCθ-mediated phosphorylation of ABCA1 is required for its association with cullin 3 in its ubiquitination and degradation. A, Raw264.7 cells were transfected with the indicated siRNA, quiesced, and treated with and without thrombin for 2 h. Cell extracts were prepared and analyzed by Western blotting for ABCA1 levels using its specific antibodies, and the blots were reprobed for siRNA target and off-target molecules to show the efficacy and specificity of the indicated siRNA. B, all of the conditions were the same as in A except that after transfection, cells were labeled with [3H]cholesterol (1 μCi/ml) for 24 h, treated with and without thrombin for 2 h, and subjected to a cholesterol efflux assay. C, Raw264.7 cells were treated with and without thrombin for the indicated time periods, and cell extracts were prepared. Equal amounts of protein from each condition were immunoprecipitated (IP) with ABCA1 antibodies; the immunocomplexes were analyzed by Western blotting for pSer/Thr or cullin 3 antibodies; and the blot was reprobed for ABCA1. D, top, cells were transfected with siControl or siPKCθ, quiesced, and treated with and without thrombin for 2 h; cell extracts were prepared; equal amounts of protein from each condition were immunoprecipitated with ABCA1 antibodies; the immunocomplexes were analyzed by immunoblotting with pSer/Thr or cullin 3 antibodies; and the blot was reprobed for ABCA1. The same cell extracts were also analyzed by Western blotting for PKCθ and β-tubulin levels to show the efficacy of the siRNA on its target and off-target molecules. Bottom, equal amounts of proteins from same the cell extracts were also immunoprecipitated with anti-ubiquitin antibodies, and the immunocomplexes were analyzed by Western blotting for ABCA1 levels using its specific antibodies. E and F, aortas from CD-fed ApoE−/− or 12 weeks of WD-fed ApoE−/− and ApoE−/−:Par1−/− mice were isolated, tissue extracts were prepared, and equal amounts of proteins from each regimen were immunoprecipitated with Par1 or ABCA1 antibodies. The anti-Par1 and anti-ABCA1 immunocomplexes were analyzed by Western blotting for Gα12 levels and pSer/Thr or cullin 3 levels, respectively. The same tissue extracts were also analyzed by Western blotting for pPyk2, pGab1, and pPKCθ levels using their phospho-specific antibodies, and the blots were normalized for their total levels. G, whole blood was collected from CD-fed ApoE−/− or 12-week WD-fed ApoE−/− and ApoE−/−:Par1−/− mice, and plasma prothrombin/thrombin levels were measured using a kit from Abcam following the manufacturer's protocol. Error bars, S.D.