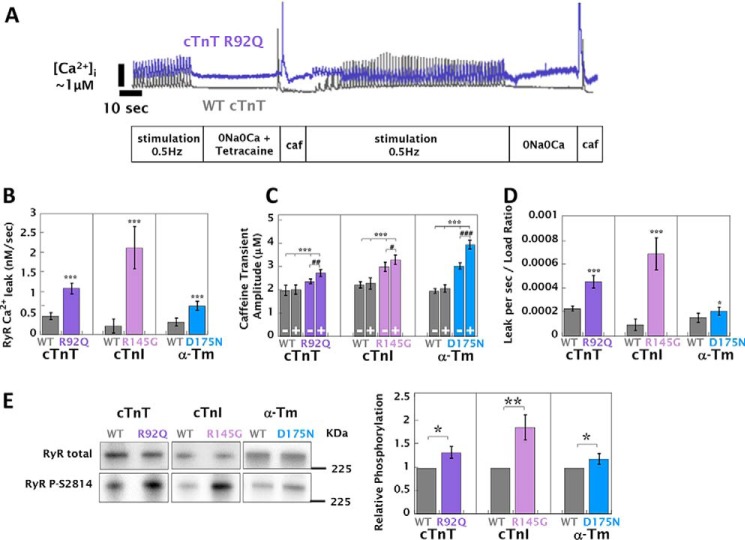
Figure 6.
HCM mutant-infected cardiomyocytes have increased RyR leak/load relationships and CAMKII activation of RyR. SR Ca2+ load and Ca2+ leak via the RyR were assessed by the sequential perfusion of 1.8 mm CaCl2 (A), Na+- and Ca2+-free solution containing 1 mm tetracaine for 50 s (B), the direct application of 10 mm caffeine (C), 1.8 mm CaCl2 for 100 s (D), Na+- and Ca2+-free solution for 50 s (E), and a direct application of 10 mm caffeine (F). A, representative experimental traces for WT cTnT (gray) and cTnT R92Q (purple) cardiomyocytes. All mutants tested were analogous to cTnT R92Q, whereas all control and WT-infected cells tested resembled WT cTnT. B, the observed RyR-dependent leak rate. C, the buffering-adjusted caffeine transient amplitude taken after perfusion with either 0Ca0Na (−) or with 0Na0Ca solution containing 1 μm tetracaine (+). D, the leak/load ratio, calculated as the leak rate divided by the caffeine transient amplitude at the end of each experiment. Each bar graph is an average of 30 cells, 15 of which were acquired as shown in A or B, whereas a further 15 were acquired by reversing the sequence of 0Na0Ca solution with or without 1 μm tetracaine to prevent errors from fura2 signal degradation and cell fatigue. A full breakdown of Δ[Ca2+]i for each perfusion switch can be found in Fig. S10, and the extracted parameters are also tabulated in Table S7. Representative Western blots of total and phosphoserine 2814 RyR are given in E, with the adjacent bar graph showing the average change in phosphorylation from densitometry measurements, all preparations were paced for 8 h at 0.5 Hz. Unpaced preparations showed no significant changes at the same site (data not shown). ***, p < 0.001; **, p < 0.01; *, p < 0.05 for comparing WT to HCM mutant. ###, p < 0.001; ##, p < 0.01; #, p < 0.05 for comparing the presence or absence of 1 mm tetracaine.